Phenylacetic Acid via the Willgerodt Reaction from Styrene/AcetophenoneIntroductionThe Willgerodt-Kindler reaction with morpholine transforms acetophenones and styrenes to phenylacetic acids, through the intermediate phenylacetothiomorpholide, which is hydrolyzed in acid or basic solution to the desired acid. Other amines will also work instead of the somewhat watched morpholine, but the yields of the reaction will suffer, see table below for the synthesis of phenylacetic acid from styrene using various amines [1], other reaction conditions as below.
Phenylacetic acid [1]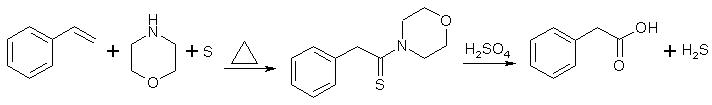
A mixture of styrene (104 g, 1.00 mole), sulfur (80g, 2.5 moles) and morpholine (174 g) was refluxed two hours (internal temp rising to about 175°C). The cooled reaction mixture was taken up in chloroform (some sulfur remained undissolved) and the chloroform solution was washed successively with an equal volume of water, sufficient dilute hydrochloric acid to remove the excess morpholine, and finally with an equal volume of water. The solvent was removed under vacuum, and the residue of crude phenylthio-acetmorpholide was refluxed for ten hours with 1200 ml of 50% (by weight) sulfuric acid. The cooled hydrolysis mixture was extracted with three 500 ml portions of ether and the combined ethereal extract was washed with 100 ml of 12% caustic soda. The caustic wash was acidified with conc. HCl and then extracted three times with 300 ml portions of ether. Removal of the solvent from the combined ethereal extract gave 114g (84% yield) of nicely crystalline phenylacetic acid, m.p. 72-74°C. One run in which the original reaction mixture was concentrated under vacuum on the steam cone and then hydrolyzed directly with 50% sulfuric acid gave only a 59% yield of phenylacetic acid, m.p. 71-74°C. p-Methoxyphenylacetic acid [2]Reflux a mixture of 42 g. of p-methoxyacetophenone, 13-5 g. of sulphur and 30 g. (30 ml.) of morpholine for 5 hours. Pour the reaction mixture slowly into water, allowing the first addition to crystallise before the bulk of the mixture is added. Filter off the crude yellow solid, grind it up thoroughly with water, filter again and dry in the air. The yield of crude acetothiomorpholide, m.p. 65-67°C, is 68g. Recrystallisation from dilute methanol raises the m.p. to 71-72°. Add 50 g. of the crude acetothiomorpholide to 400 ml. of 10% alcoholic sodium hydroxide solution and reflux the mixture for 10 hours. Distil off most of the alcohol, add 100 ml. of water to the residue, and strongly acidify the alkaline solution with hydrochloric acid. Cool, extract thrice with ether, dry the combined ether extracts, evaporate the solvent, and recrystallise the residue from water or dilute alcohol. The yield of p-methoxyphenylacetic acid, m.p. 85-86°, is 26 g. A further quantity of acid may be obtained by extracting the mother liquors with ether. 3,4,5-trimethylphenylacetic [2]A mixture of 48.6 g of 3,4,5-trimethylacetophenone, 39 g of redistilled morpholine, and 14.4 g of sulfur was reflused for 12 hr. The warm reaction mixture was poured into 175 ml of hot ethanol and allowed to cool to permit the product to crystallize; yield, 62.6 g (79%) of 3,4,5-trimethylphenylacetothiomorpholide, mp 120-122 C, sufficiently pure for the next step. A sample recrystallized from ethanol melted at 123-124 C. 3,4,5-trimethylphenylacetic acid. A mixture of 51 g of 3,4,5-trimethylphenylacetothiomorpholide, 110 ml of acetic acid, 16 ml of sulfuric acid, and 25 ml of water was heated under reflux for 5 hr and decanted from the small amount of tar formed into 850 ml of water with stirring. The precipitated crude product was collected, washed with water, and heated with 225 ml of 5% aqueous sodium hydroxide. Filtration from a small amount of insoluble matter and acidification with dilute hydrochloric acid gave 30 g (88%) of 3,4,5-trimethylphenylacetic acid sufficiently pure for the next step. A sample recrystallized from benzene-petroleum ether melted at 125-126 C.Morpholine From Diethanolamine [3]2 moles of diethanolamine is placed in a flask with a thermometer and an air-cooled reflux condenser, and aqueous HCl is added until slightly acid. Heat rapidly to drive off all water, and keep the temperature at 200-210°C for 15h. Let cool, and add an excess of calcium oxide and dry distill the mixture. The distillate is first dried over NaOH pellets, then refluxed over small pieces of sodium for 30 min, and then fractionally distilled, bp 126-129°C. The yield is around 50% of theory. References [1] JACS 68, 2335 (1946)
|