Reduction of 2,4,5-Trimethoxyphenyl-2-nitropropene to TMA-2by Antibody2Have been been investigating a few different nitropropene reductions recently. There isn't alot available in terms of procedures, so I will post these. Reduction of TMP2NP using NaBH4 in THF/IPA followed by Al/Hg in aqueous MeOH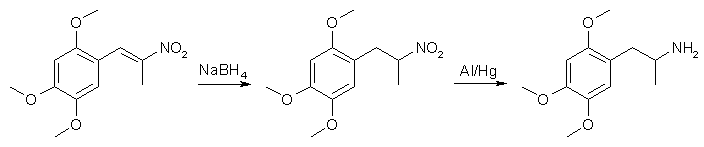
An oven-dried 250ml rb flask sitting in an ice/salt bath is charged with 50ml IPA (dried w/ MgSO4), 1g 8-20 mesh indicating silica gel and 80mmol NaBH4 (3g) with mag stirring. When temp of solution was 0°C a solution of 20mmol of desiccated TMP2NP (5g) in 100ml dry THF, is added dropwise from a pressure equalized addition funnel over 1.5 hours. Rxn is allowed to stir for an additional 0.5 hours, until all traces of orange had disappeared. Ice bath is removed and rxn mixture was vacuum filtered and the solvent stripped off using low vac on a water bath. 150ml MeOH is added to the residue followed by the slow addition of 320mmol GAA (19g) from a pressure equalized addition funnel vented by a hose out of doors. The funnel is removed and 15ml dH2O is added with 50mg HgCl2. With vigourous mag stirring 0.5 moles Al (13.4g) (regular reynolds wrap) is slowly added over a 2 hour period and allowed to stir an additional hour while remaining Al goes into suspension. Rxn is transferred to a 500ml erlenmeyer flask and slowly basified with 50% NaOH. When rxn with remaining Al has subsided, it is a viscous gel. Flask is stopped and shaken until the gel breaks down into a fluid again. It is then extracted 2X with 100ml toulene. Extracts pooled and washed 1X w/ Saturated NaCl soltion, 1x w/ dH2O, dryed over MgSO4. Then pre-gassed aliquots of toulene are added with filtration between aliquots until no more crystals precipitate. Yield: 11mmol TMA2.HCl (2.75g) 55% molar yield Reduction of TMP2NP using Uruishibara Catalyst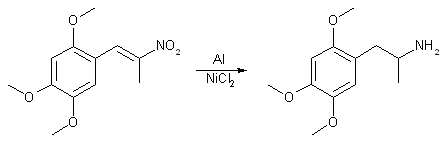
Catalyst prep To a solution of 1ml 31% HCl, 40g NiCl2.6(H20) in 350ml EtOH in a 500ml beaker at 50°C is added 35g of shredded Al (regular reynolds wrap) slowly over a 3.5 hour period, when evolution of hydrogen had ceased the the rxn was a viscous gel. This was placed in a 4l beaker and rinsed several times with tap water, each time allowing suspension to settle before decanting. This precipitated nickel was air dried overnight on a filter paper (Note: larger pieces of foil were removed). Activation 10g of the above catalyst was placed in a beaker containing 385 ml 40% aqueous AcOH and 89g NaCl at 70°C for 7 minutes, then the solution is decanted and the Nickel rinsed with 60°C dH2O, then rinsed with EtOH then placed in a 500ml erlenmeyer flask containing 250ml EtOH and charged with 20mmol TMP2NP (5g). With moderate overhead stirring, 10g of Al is added 1g at a time followed by a 3ml aliquot of 31% HCl with each addition. Addition takes 2 hours, it is then allowed to stir an additional hour while evolution of hydrogen subsides. Rxn become one viscous gel to which and 100ml EtOH is added with stirring. Rxn is then slowly basified with 50% NaOH. After AlO has settled the alcoholic overhead is decanted and the sludge extracted with 100ml toulene. The EtOH is stripped off using low vac on a water bath, the residue being taken up with the toulene extract. Extract was washed 1x w/ Saturated aqueous NaCl and 1x with dH2O. This is dried over MgSO4. Pre-gassed toulene is added in small aliquots with precipitated crystals being vac filtered between aliquots. Yield: 10mmol TMA2.HCl (2.5g) 50% Molar yield Reduction of 2,4,5-trimethoxyphenyl-2-nitropropene to 2,4,5-trimethoxyphenyl-2-propanoneA mixture of Glacial Acetic Acid (30ml) and 20-mesh iron (14g, 0.24 mol) in a 250 ml three-necked RBF equipped with a condenser, heating mantle and mechanical stirrer was vigorously stirred and heated at reflux until the mixture became greyish-white (about 30 min). A solution of 2,4,5-trimethoxyphenyl-2-nitropropene (6g, 24 mmol) in glacial acetic acid was added dropwise to this rapidly stirred solution. The reaction mixture was heated under reflux for a total of 3h. The resulting grayish-dark green mixture was vacuum filtered through a bed of celite and then washed with hot acetic acid. The filtrate was then diluted with 100 ml water and extracted with 3x50ml DCM. The combined organic extracts was washed with 5% NaHCO3 and water, dried over MgSO4 and evaporated to give a brown oil. Sublimation at 115-120°C (0.5 mmHg) gave 4g (75%) of the ketone, mp 44-46°C. Reference: J. Med. Chem. 23, 1318-1323 (1980) |