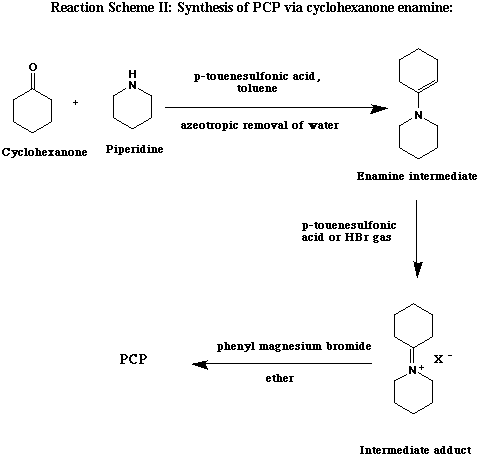
This method is used less frequently in underground synthesis, but has the advantage of not involving toxic cyanide compounds. The first step of the reaction is the dehydration of piperidine and cyclohexanone to form an enamine. The addition of anhydrous p-toluenesulfonic acid or dry HBr produces an intermediate imminium salt. Reaction of this salt with phenylmagnesium bromide yields PCP. This method is most applicable to cyclic secondary amine analogs such as piperidine, pyrrolidine, or morpholine, rather than an acyclic N-substituent such as ethyl or dimethyl (ref. 8, 12 ).
This route has an overall yield of ~70%, with a difficulty rating of 3 out of 10, and a hazard rating of 2 out of 10 (ref. 64).

Step 1. Preparation of cyclohexenyl piperidine: A solution of 98 g (1.0 mole) of cyclohexanone, 100 g (1.17 mole) of piperidine, and 2 g (0.0105 mole) of p-toluenesulfonic acid in 300 ml of toluene is refluxed under azeotropic distillation conditions until the evolution of water stops (about 13 hours). The best method for this is to use a Dean Stark or Barrett water trap, but if one is not available, it may be improvised with a distillation head as described in Vogel's Textbook of Practical Organic Chemistry.
Either p-toluenesulfonic acid or dry HBr gas can be used in the next step. The intermediate cyclohexenyl-piperidine (enamine) from the previous step is best used crude, but it can be distilled with adequate vacuum.
Step 2, method A. Use of p-toluenesulfonic acid: 190 g of p-toluenesulfonic acid monohydrate in 250 ml of toluene is heated under a Dean Stark trap until all the water is removed. This is then added to 165 g of cyclohexenyl-piperidine in 500 ml of ether with ice cooling to keep temp at 0 deg C. A solution of 1 mole of phenylmagnesium bromide is prepared as in Scheme I from 157 g of bromobenzene and 24 g of Mg turnings in 750 ml of ether. This is added to the cyclohexenyl piperidine while holding the temp at 0 to 5 deg C. The mixture is stirred for an additional 30 min after the dropwise addition is complete.
A mixture of saturated aqueous ammonium chloride and ammonium hydroxide is added to neutralize the excess phenylmagnesium bromide (approx. 100 gm of NH4Cl and 20 ml of strong NH40H in enough water to make a saturated solution). The ether layer is then removed in a separatory funnel, dried by the addition of potassium carbonate, filtered, and evaporated to give PCP. This can be converted to the hydrochloride salt by dissolving it in an excess of isopropanol saturated with HCl gas and precipitating with ether, followed by crystallization from a mixture of ether and isopropanol.
Step 2, method B. Use of HBr gas: The reaction mixture from step one is diluted to 2 L with dry toluene and dry HBr gas was bubbled through until the solution is acidic. The resulting slurry is added at once to a cold (5 deg. C) stirrred solution of phenylmagnesium bromide prepared from 236 g (1.51 moles) of bromobenzene and 38 g of Mg (1.56 moles) in 1 L of dry ether. The temperature will rise to 45 C and the mixture should be stirred for an additional 30 minutes.
300 ml of 48% aqueous HBr is then added, producing the HBr salt of PCP, which is filtered from the reaction mixture. The crude HBr salt is then basified with NaOH, extracted with hexanes, toluene, etc., and either evaporated to yield PCP freebase or treated with isopopanol saturated with HCL, followed by dilution with ether to give PCP hydrochloride.
Next Section: Synthesis of PCP via imines Back to Contents