Preparation of Ketones from Grignard Reagents and Acetic Anhydride[1][ Back to the Chemistry Archive ]We have found that excellent yields of methyl ketones may be obtained by the addition of Grignard reagents to an ether solution of acetic anhydride at about -70°C. Primary, secondary, tertiary aliphatic, and aromatic Grignard reagents give 70-79% yields of the corresponding methyl ketones while the allyl and benzyl reagents give 42 and 52%, respectively[2]. We attribute the success of these reactions at low temperature to the thermal stability of the complex formed by the addition of one molecule of Grignard reagent to one of the carbonyl groups of acetic anhydride, and to its decreased solubility. These factors both tend to reduce the further reaction of the complex with more Grignard reagent to form the tertiary alcohol. At the low temperature involved there is probably no cleavage of this complex to form ketone which might further react. 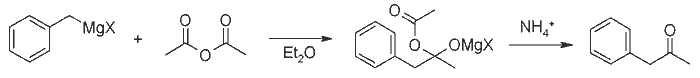 ExperimentalIn a typical experiment, 0.2 mole of a titrated Grignard reagent was added slowly during one hour to a stirred solution of 40g of acetic anhydride in 100ml of dry ether in a 500ml 3-necked flask cooled by a mixture of Dry Ice and acetone in a Dewar flask. The added reagent was cooled by dripping through a tube externally cooled with Dry Ice. After stirring for two to three hours the cooling bath was removed and the mixture was treated with ammonium chloride solution. After washing out the acetic anhydride and acid with alkali the ether was fractionated and the ketones distilled. For the most part the ketones were identified by boiling point and index of refraction, although a few derivatives were made. The following Grignard reagents gave the corresponding methyl ketones in the following yields: n-butylmagnesium chloride, 79%; n-butylmagnesium bromide, 79%; s-butylmagnesium bromide, 78%; t-butylmagnesium chloride; 77%; phenylmagnesium bromide, 70%; benzylmagnesium chloride, 52%; and allylmagnesium bromide, 42%. With phenylmagnesium bromide and propionic anhydride a 59% yield of propiophenone was obtained. References [1] Newman & Booth, J. Am. Chem. Soc. 67, 154 (1945)
|