Abstract
Safrole and eugenol isomerization reactions were carried out under microwave irradiation at atmospheric pressure and homogeneous medium with various alcohols used as solvents and different base concentrations. The rate reaction enhancement shown was 9.3 to 13.9 times faster than conventional reflux.
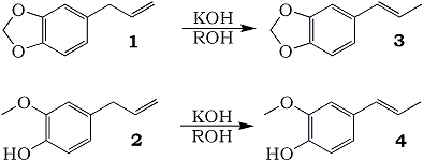
The safrole (1) and eugenol (2) isomerization reactions are of great interest, since the products isosafrole (3) and isoeugenol (4) are used industrially in the process of manufacturing pharmacos and fragrances. Safrole and eugenol are compounds which are easily obtained, once they are the main components in sassafras and clove oils, respectively1.
Attempts to isomerize these compounds to the more stable alkenes (3) and (4) by methods described in the literature2-4 were frustrating due to high base and temperature conditions, inconvenient for large scale bench preparations. Among the alternative methods we tried is the use of microwave irradiation.
Table I:
A comparison of times and yields in isomerization reactions
of safrole using microwave and conventional heating.
The results are the mean of three experiments.
| Solvent | [KOH] | t(c)a | Yield | t(mw)b | Yield | t(c)a/t(mw)b |
| EtOH | 2M |
25 h |
88% |
240 min |
90% |
6.2 |
| EtOH | 4M |
5 h |
98% |
30 min |
99% |
10.0 |
| EtOHc | 4M |
4 h |
99% |
20 min |
99% |
12.0 |
| 1-PrOH | 2M |
4 h |
95% |
60 min |
95% |
4.0 |
| 2-BuOH | 2M |
3 h |
95% |
60 min |
96% |
3.0 |
| 1-BuOH | 2M |
1 h |
98% |
20 min |
99% |
3.0 |
| 1-BuOH | 4M |
0.25 h |
99% |
3 min |
99% |
5.0 |
a) Reaction time using conventional heating
b) Reaction time using microwave heating
c) KCl 2M present.
To study the microwave irradiation effect over isomerization reactions of safrole and eugenol at atmospheric pressure and homogeneous medium, experiments with various alcohols used as solvents and different base concentrations (Tables I and II) were carried out in an adapted microwave oven (Scheme I) and in conventional reflux.
The results showed that the isomerization reactions were fastest for safrole in 1-butanol5 and for eugenol in glycerol, using either the method of microwave irradiation or conventional reflux.
The reactions carried out under microwave irradiation were 2.7 to 13.2 times faster than in conventional reflux. This ratio between the reaction times in conventional reflux and microwave irradiation - t(c)/t(mw) - under the same conditions, quantifies the microwave heating effect.
Table II:
A comparison of times and yields in isomerization reactions
of eugenol using microwave and conventional heating.
The results are the mean of three experiments.
| Solvent | [KOH] | t(c)a | Yield | t(mw)b | Yield | t(c)a/t(mw)b |
| EtOH | 2M |
25 h |
85% |
480 min |
87% |
3.1 |
| EtOH | 4M |
5 h |
95% |
60 min |
96% |
5.0 |
| 1-BuOH | 4M |
2 h |
98% |
40 min |
97% |
3.0 |
| 1-Pentanol | 4M |
1.2 h |
98% |
25 min |
99% |
2.7 |
| Ethanediol | 4M |
1 h |
99% |
10 min |
98% |
6.0 |
| Glycerol | 4M |
0.75 h |
98% |
3 min |
98% |
13.2 |
a) Reaction time using conventional heating
b) Reaction time using microwave heating
The ratio shows a relationship with the solvent static dielectric permittivity (Table III), also called "dielectric constant" (in which the losses angle tangent is dependent6,7). The largest ratios were in ethanol to safrole isomerization and in glycerol to eugenol isomerization, the solvents with highest dielectric permitivities.
In the isomerization reactions, a large ratio relationship with ionic species concentration was still observed (the ionic species increase the dielectric-loss tangent8-10). The reaction ratio of safrole isomerization in ethanol and 2M KOH was 6.2 times. In ethanol and 4M KOH the ratio was 10.0 times, and it was amplified to 12.0 times with the presence of 2M KCl.
Table III:
Static dielectric permittivity - E(s) - of compounds
used in isomerization reactions.
| Solvents | E(s)a | Reactants/Products | E(s) |
| Ethanol | 24.5 | Safroleb | 3.0 |
| 1,2-Ethanediol | 38.7 | Isosafroleb | 3.4 |
| 1-Propanol | 20.3 | Eugenolb | 9.7 |
| Glycerol | 42.5 | Isoeugenolb | 9.8 |
| 1-Butanol | 17.5 | ||
| 2-Butanol | 16.6 | ||
| 1-Pentanol | 13.9 | ||
a) Tabulated values11 at 25°C.
b) Measured at 25°C on a Dekameter DK03
apparatus.
The results demonstrate that the microwave energy absorption by reactional solutions was due to conductivity effects (conductivity loss), commonly known as "ohmic effects", together with the dipole alignment and realignment losses of polar molecules (dipolar loss), mainly by solvent molecules.
The cis/trans proportion isomers (30/70) were the same for safrole and eugenol isomerization in microwave heating and in conventional reflux12.
The enhancement reaction rate of safrole and eugenol isomerizations, shown under microwave irradiation (2.45 GHz/500W) at homogeneous medium and atmospheric pressure, was caused by localized superheating (temperature gradients) in the reactional solutions, as reported elsewhere10,13-15. These hot spots result from the transferred kinetic energy in and unhomogeneous way, characterised by the occurrence of carbonisation.
When these results are amplified to industrial scales, involving significant differences of time and energy savings, the relationship between costs and advantages of this industrial process may well be significantly altered, resulting in successful applications.
Scheme I
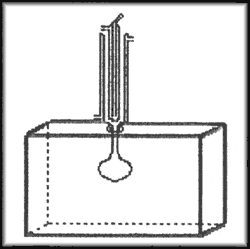
General Procedure:
The alcoholic alkaline solution is prepared by prolonged stirring of 8.8g (or 4.4g) of KOH pellets in 30 ml of alcohol (Tables I and II). The KOH and the alcohols were of analytical purity grade.
The alkaline solution is placed in a round-bottom flask provided with a reflux condenser (microwave and conventional systems). Then 4.0g of safrole (or eugenol) is added and the solution heated. The products arising are followed by UV-Visible spectroscopy by aliquot withdrawal, and, after isolation, the products are characterised by 1H-NMR spectroscopy16.
Apparatus: The microwave oven used for this study was a 500W Continental 2001. The reflux system was confectioned in Pyrex glass and adapted through a hole in the oven's upper wall, having a diameter smaller than half the wavelength of the radiation to avoid leakage of microwave radiation10.