Grignard Reactions of Nitriles in Benzeneby Bombard et al, Tet Lett 21, 155-158 (1980)OCR, graphics and french translation by Rhodium [ Back to the Chemistry Archive ] Compared to the corresponding carbonyl compounds, the nitriles are substrates not very reactive with etheral organomagnesium solutions; they do not readily form the desired ketones, and the reactions oftern give low yields. To increase their reactivity, several authors [1] have used toluene as the reaction solvent, and higher temperatures could be achieved (reflux of the toluene). The increase in the obtained yields was attributed to the higher temperature and not to the reactivity of the organomagnesium species in this solvent [2]. In the present communication, we describe the first results of our performed reasearch of nitriles at ambient temperature, with the Grignard reagent, prepared in benzene in the presence of one equivalent of diethyl ether (table 1).
The results in this table show, in fact, that the yields of ketones in the investigated reactions in benzene are superior to 80%, while they do not exceed 50% in diethyl ether at the same temperature. A known exception has be signaled: the acetonitrile with benzylmagnesium chloride furnishes less than 50% of the corresponding ketone [3]. Besides, as It is well known [4] that using an excess quantity of organomagnesium reagent to a nitrile gives primary amines as a side product. The mechanism of the nucleophilic addition of the Grignard reagents to the nitriles [5] in diethyl ether can be simplified in the following manner: 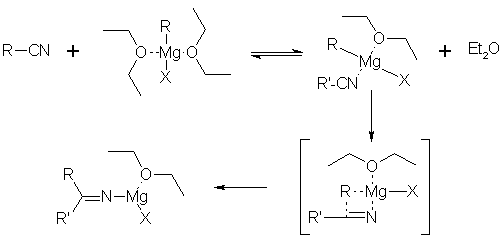
The formation of the complex supposes the coordination of the magnesium of the organomagnesium reagent with the nitrile and consequently, the displacement of one equivalent of solvent, in the case of diethyl ether, that play a role determining in the sequence of the reaction. Besides, it should be remembered that the different structure of the organomagnesium reagent in benzene compared with ether, is not negligible. In fact, in ether, the chlorides of the alkylmagnesium behave as dimers in the used concentrations while the bromides and the iodides change structure according to the concentration. They are monomers at a concentration 0.5 M and dimers at a concentration of 0.5-1.5 M [7]. On the contrary, in the aromatic solvents, and in the presence of one equivalent of ether, the reactivity of the Grignard, whatever the halogen anion, present themselves as dimers at low concentrations [8]. Consequently, we postulate the hypothesis that the increase in yields in the benzene is attributable to the reactivity of the organomagnesium reagent in this solvent, probably due to the facts that the partial desolvation of the reagent and the replacement of a molecule ether by a nitrile molecule as a coordinant of the magnesium, making the reaction go more easy (equation 2). 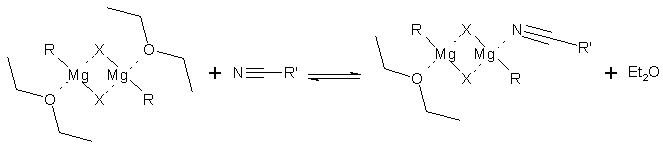
In addition, it has been observed that with reactions of organolithiums with enolizable nitriles that solvation by polar solvents favor the reaction of the enolisation [10], and not the mode of addition. The new method, that is the focus of this work, rest on the preparation of the organomagnesium reagent in hydrocarbon solvents and their subsequent usage with the nitriles is of important value both from an academic or practical viewpoint. We have actually observed that during extended heating of refluxing toluene, added to the reaction condensation and to the one of formation of the substituted amines another: 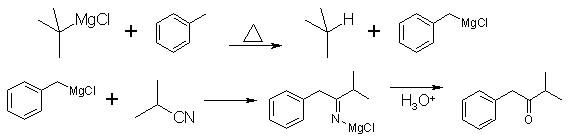
Thus at the time of the addition of the tert-butylmagnesium chloride, prepared according to the method of Leigh [11] (in toluene, in the presence of one equivalent of THF), one has isolated an aromatic ketone (28% by GC) instead of the anticipated trimethyl-2,2,4-pentanone. Spectroscopic and physical data identifies it without ambiguity as phenyl-1-methyl-3-butanone-2 [12]. The same ketone (phenyl-1-methyl-3-butanone-2) has been obtained (18% by GC) while refluxing isobutyronitrile with the same grignard reagent, prepared in ether, but the solvent replaced by toluene after evaporation. One notes that the increase of polarity of the solvent favor, even at that temperature, the transformation of the tert-butylmagnesium chloride into the benzylic counterpart. At this stage, we do not have verified if the mechanism of this last reaction does effectively intervene with a single electron transfer [13], but it imported to note it as another parallel secondary reaction to the one already described. References [1] Kharasch et al. Grignard reactions of Nonmetallic Substances, PrenticeHall, New York (1954) pp. 769-772.
|
||||||||||||||||||||||||||||||