Summary:
A variety of aromatic compounds have been converted to aldehydes via a facile formylation process employing hexamethylenetetramine and trifluoroacetic acid.
In the Duff reaction,1-3 hexamethylenetetramine (HMTA) is employed, usually with glyceroboric acid, to convert highly activated aromatic compounds to their formyl derivatives. The process is quite limited in scope, having been most widely used in the conversion of phenols to o-hydroxybenzaldehyde derivatives. The required conditions are rigorous, and the yields are generally low.
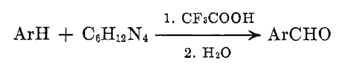
When the hexamethylenetetramine is used in conjunction with trifluoroacetic acid, a variety of aromatic compounds, including simple hydrocarbons, can be converted to imine products which are transformed to the aryl aldehydes on hydrolysis (eq 1). The required conditions are mild, and good yields of pure products can be easily isolated. A high order of para regioselectivity is exhibited.
Experimental
Thus, a mixture of 12.2 g of 2,6-xylenol (100 mmol), 14.0 g of hexamethylenetetramine (100 mmol), and 150 ml of trifluoroacetic acid was heated at reflux (83-90°C) for 12 hr. The products were concentrated and combined with 600 ml of ice water; the resultant mixture was stirred for 15 min, made basic with Na2CO3, and extracted with ether. Evaporation of the ether solution left a yellow solid which was recrystallized from chloroform-pentane to afford 14.3 g (95% yield) of 3,5-dimethyl-4-hydroxybenzaldehyde, mp 111-112.5°C (lit.4 mp 113-114°C).
The data for a number of these transformations are summarized in Table I.
Table I
Formylation of Aromatics by HMTA in Trifluoroacetic Acid
| Substrate | HMTA:Substrate |
Product(s) |
| tert-Butylbenzene | 1:1 |
p-tert-Butylbenzaldehyde (75%) |
| p-Xylene | 1:1 |
2,4-Dimethylbenzaldehyde (55%) |
| Toluene | 1:1 |
p-Tolualdehyde (50%) o-Tolualdehyde (11%) |
| Benzene | 1:4 |
Benzaldehyde (32%) (Sealed tube, 125-150°C) |
| 2,6-Dimethylanisole | 2:1 |
3,5-Dimethyl-4-Methoxybenzaldehyde (74%) |
| Benzo(1,4)dioxane | 1:1 |
4'-Formylbenzo(1,4)dioxane (37%) 3'-formylbenzo(1,4)dioxane (2%) |
| Diphenyl ether | 2:1 |
p-Phenoxybenzaldehyde (29%) 4,4'-diformyldiphenyl ether (25%) |
| 2,6-Di-tert-butylphenol | 1:1 |
3,5-bis(tert-Butyl)-4-hydroxybenzaldehyde (60%) |
| 2,6-Xylenol | 1:1 |
3,5-Dimethyl-4-hydroxybenzaldehyde (95%) |
Methylimine derivatives are immediate precursors of the aldehydes. When the reaction products derived from toluene were subjected to rapid hydrolytic workup, the p- and o-toluimines CH3C6H4CH=N-CH3 were obtained in predominance to the carbonyl compounds. Whether such products are formed by rearrangement of the methyleneimines ArCH2N=CH2 or arise in exchange reactions involving methylamine remains to be determined.
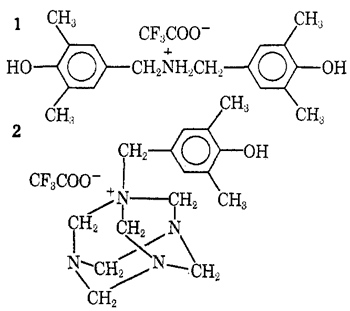
Other kinds of intermediates are isolable when both heating and hydrolysis are avoided. Thus, the 2,6-xylenol-hexamethylenetetramine-trifluoroacetic acid system when kept below 30°C for 3 hr yielded a complex mixture from which the dibenzylammonium salt5 1 (41%) and the hexaminium salt6 2 (15%) were isolated after evaporation of the acid and fractional crystallization of the residue from acetonitrile-ether. No unalkylated 2,6-xylenol was recovered. The formation of 2 makes evident the relationship of this process to the Sommelet7 and Delépine8 reactions, both of which are based on transformation of N-benzyl derivatives of hexamethylenetetramine. Experiments with the trifluoroacetic acid system aimed at an illumination of these mechanistically obscure9 facets of hexamethylenetetramine chemistry are in progress.