This article is a kind of supplement to PIHKAL, since its covers the syntheses of some fluoro- and nitro analogs of 2,4,5-Trimethoxyamphetamine (TMA-2, Pihkal #158) and 2-Methoxy-4,5-Methylenedioxyamphetamine (MMDA-2, Pihkal (#133).
2-Nitro-4,5-dimethoxyamphetamine (6-Nitro-3,4-DMA)
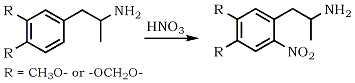
A solution of 3,4-Dimethoxyamphetamine (3,4-DMA) (1.95 g, 10 mmol) in 2N HNO3 (10 mL) was added with stirring at 15°C to 30 mL conc. HNO3 (70%, 1.42 g/mL) diluted with water (12 mL). The solution was stirred for 3 h at RT, then poured into ice-water. The precipitate was suspended in 0.1 M NaOH, the solution extracted with CH2Cl2, the extracts dried (Na2SO4) and the solvent evaporated. The residue was dissolved in toluene and dry HCl gas was bubbled through it to give 1.9 g (69%) of 6-Nitro-3,4-DMA·HCl.
2-Nitro-4,5-Methylenedioxyamphetamine (6-Nitro-MDA)
Prepared in a similar way as described above, using 3,4-Methylenedioxyamphetamine (MDA). Yield ca 80%.
5-Fluoro-2,4-Dimethoxybenzaldehyde
Phosphorus oxychloride (15.3 g, 0.1 mol) and N-methyl-formanilide (13.5 g, 0.1 mol) was stirred for 30 minutes at 25°C. Add slowly 2,4-dimethoxyfluorobenzene (15.6 g, 0.1 mol). After addition is complete, let react for 3 hours at 35°C, leave overnight and pour in ice-water. Filter precipitate and dry to give 17.7 g of the benzaldehyde (96% yield)
1-(5-fluoro-2,4-dimethoxyphenyl)-2-nitropropene
A mixture of 2-fluoro-4,5-dimethoxybenzaldehyde (4 g, 0.02 mol) and ammonium acetate (0.38 g, 5 mmol) in nitroethane (21 mL) was heated for 3 hrs at 80°C. Excess solvent was evaporated and the oily residue scratched with cold EtOH to precipitate the nitropropene (3.6 g, 69% yield)
5-Fluoro-2,4-Dimethoxyamphetamine (5-F-2,4-DMA)
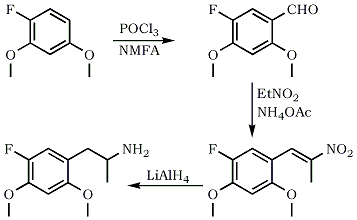
To a suspension of LAH (0.8 g, 0.02 mol) in dry THF (10 mL) was added with stirring a solution of the above nitropropene (1 g, 4.1 mmol) in dry THF (15 mL). The resulting mixture was refluxed for 3 hrs. It was then cooled and excees LAH was decomposed by adding water. After filtration and washing the inorganic precipitate with Et2O, combined extracts were evaporated and the oily residue redissolved in 0.1 N H2SO4. Wash with ether, basify and extract with CH2Cl2. Evaporate the solvent and convert the free base to its hydrochloride salt by bubbling dry HCl gas through an ethereal solution of the base to get 0.6 g (58% yield) of the isopropylamine.