Introduction
After development with the mobile phase, the plate is dried in a fume hood and heated to completely evaporate the mobile phase. Separated compounds are detected on the layer by viewing their natural color (e.g., plant pigments, food colors, dyestuffs), natural fluorescence (aflatoxins, polycyclic aromatic hydrocarbons, riboflavin, quinine), or quenching of fluorescence. Substances that cannot be seen in visible or ultraviolet (UV) light must be visualized with suitable detection reagents to form colored, fluorescent, or UV-absorbing compounds by means of derivatization reactions (postchromatographic derivatization).
Although dependent on the particular analyte and the detection method chosen, sensitivity values are generally in the nanogram range for absorbance and picogram range for fluorescence. Detection may be obtained by prechromatographic derivatization, either in solution prior to sample application or directly on the plate at the origin. As an example, dansyl derivatives of amino acids are detected directly by their fluorescence. Prechromatographic derivatization may enhance compound stability or chromatographic selectivity as well as serving for detection.
Direct Detection
Compounds that are naturally colored are viewed directly on the layer in daylight, whereas compounds with native fluorescence are viewed as bright zones on a dark background under UV light. Viewing cabinets or boxes incorporating short-wave (254 nm) and long-wave (366 nm) UV-emitting mercury lamps are available for inspecting chromatograms in an undarkened room.
Fluorescence Quenching
Compounds that absorb around 254 nm, including most compounds with aromatic rings and conjugated double bonds and some unsaturated compounds, can be detected on an “F layer” containing a phosphor or fluorescent indicator (often zinc silicate). When excited with 254-nm UV light, absorbing compounds diminish (quench) the uniform layer fluorescence and are detected as dark violet spots on a bright green background. Detection by natural fluorescence or fluorescence quenching does not modify or destroy the compounds, and the methods are therefore suitable for preparative layer chromatography. Derivatization reactions modify or destroy the structure of the compounds detected, but they are often more sensitive than detection with UV radiation.
Universal Detection Reagents
Universal reactions such as iodine absorption or spraying with sulfuric acid and heat treatment are quite unspecific and are valuable for completely characterizing an unknown sample. Absorption of iodine vapor from crystals in a closed chamber produces brown spots on a yellow background with almost all organic compounds except for some saturated alkanes. Iodine staining is nondestructive and reversible upon evaporation, whereas sulfuric acid charring is destructive. Besides sulfuric acid, 3% copper acetate in 8% phosphoric acid is a widely used charring reagent. The plate, which must contain a sorbent and binder that do not char, is typically heated at 120–130°C for 20–30 min to transform zones containing organic compounds into black to brown zones of carbon on a white background. Some charring reagents initially produce fluorescent zones at a lower temperature before the charring occurs at a higher temperature.
Selective Detection Reagents
Selective reagents form colored or fluorescent compounds on a group- or substance-specific basis and aid in compound identification. They also allow the use of a thin-layer chromatography (TLC) system with less resolution because interfering zones will not be detected. Examples include the formation of red to purple zones by reaction of α-amino acids with ninhydrin, detection of acidic and/or basic analytes with the indicator bromocresol green, and location of aldehydes as orange zones after reaction with 2,4-dinitrophenylhydrazine hydrochloride.
Certain specialized reagents are used to visualize compounds based on their biological activity. Cholinesterase-inhibiting pesticides (e.g., organophosphates, carbamates) are detected sensitively by treating the layer with the enzyme and a suitable substrate, which react to produce a colored product over the entire layer except where colorless pesticide zones are located due to their inhibition of the enzyme–substrate reaction.
Application of Detection Reagents
Chromogenic and fluorogenic liquid detection reagents are applied by spraying or dipping the layer. Various types of aerosol sprayers that connect to air or nitrogen lines are available commercially for manual operation. For safety purposes, spraying is carried out inside a laboratory fume hood or commercial TLC spray cabinet with a blower (fan) and exhaust hose, and protective eyeware and laboratory gloves are worn. The developed, dried plate is placed on a sheet of paper or supported upright inside a cardboard spray box. The spray is applied from a distance of about 15 cm with a uniform up-and-down and side-to-side motion until the layer is completely covered. It is usually better to spray a layer two or three times lightly and evenly with intermediate drying rather than a single, saturating application that might cause zones to become diffuse. Studies are required with each reagent to determine the optimum total amount of reagent that should be sprayed, but, generally, the layer is sprayed until it begins to become translucent. After visualization, zones should be marked with a soft lead pencil because zones formed with some reagents may fade or change color with time.
Dipping is usually the best method for uniform reagent application and will produce the most reproducible results in quantitative densitometric analysis. The simplest method is to manually dip for a short time (5–10 s) in a glass or metal dip tank. A more uniform dip application of reagents can be achieved by the use of a battery-operated automatic, mechanical chromatogram immersion instrument (Fig. 1), which provides selectable, consistent vertical immersion and withdrawal speeds between 30 and 50 mm/s and immersion times between 1 and 8 s for plates with 10- or 20-cm heights.
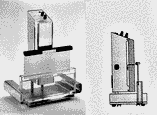
Fig. 1
Camag chromatogram immersion device set for a 10-cm dipping depth with high-performance
TLC plates. Vertical dipping and removal rates and the residence time in the reagent can be preselected.
The immersion device can also be used for impregnation of layers with detection reagents prior to initial zone application and development and for postdevelopment impregnation of chromatograms containing fluorescent zones with a fluorescence enhancememt and stabilization reagent such as paraffin. Dip application to the layer cannot be used when two or more aqueous reagents must be used in sequence without intermediate drying, such as the detection of primary aromatic amines as pink to violet zones by diazotization and coupling with the Bratton–Marshall reagent composed of sodium nitrite and N-(1-naphthyl) ethylenediamine dihydrochloride aqueous spray solutions. Dip reagents must be prepared in a solvent that does not cause the layer to be removed from the plate or the zones to be dissolved from the layer or to become diffuse.
Heating the Layer
Layers often require heating after applying the reagent in order to complete the reaction upon which detection is based and ensure optimum color development. Typical conditions are 10–15 min at 105–110°C. If a laboratory oven is used, the plate should be supported on a solid metal tray to help ensure uniform heat distribution. A plate heater (Fig. 2), which contains a 20×20-cm flat, evenly heated surface, a grid to facilitate proper positioning of TLC and high-performance TLC plates, programmable temperature between 25°C and 200°C, and digital display of the programmed and actual temperatures, will usually provide more consistent heating conditions than an oven. Prolonged heating time or excessive temperature can cause decomposition of the analytes and darkening of the layer background and should be avoided.

Fig. 2
Camag TLC plate heater.
Some reagents can be impregnated into the layer before spotting of samples if the selectivity of the separation is not affected. Detection takes place only upon heating after development. This method has been used for the detection of lipids as blue spots on a yellow background on silica gel layers preimpregnated with phosphomolybdic acid. A few detection reagents (HCl, sulfuryl chloride, iodine) can be transferred uniformly to the layer as vapors in a closed chamber rather than as solutions.
Zone Identification and Confirmation
The identity of the detected TLC zones is obtained initially by comparison of characteristic Rf values between samples and reference standards chromatographed on the same plate; Rf equals the migration distance of the center of the zone from the start (origin) divided by the migration distance of the mobile phase front from the start. Identity is more certain if a selective chromogenic detection reagent yields the same characteristic color for sample and standard zones. Because the chromatogram is stored on the layer, multiple compatible, specific detection reagents can be applied in sequence to confirm the identity of unknown zones. As an example, almost all lipids are detected as light green fluorescent zones by use of 2,7-dichlorofluorescein reagent, and absorption of iodine vapor differentiates between saturated and unsaturated lipids or lipids containing nitrogen. The identity of zones is confirmed further by recording UV or visible absorption spectra directly on the layer using a densitometer (in situ spectra) or by direct or indirect (after scraping and elution) measurement of Fourier transform infrared, Raman, or mass spectra.