Cheap Home-Made Gloveboxby Jeff T. Suri Journal of Chemical Education Vol. 78(11), 1513 (2001)[ Back to the Chemistry Archive ] The Construction of the UCSC Econo-Box: An Inexpensive Yet Effective Glove Box The Journal of Chemical Education has presented articles describing the construction of inexpensive yet effective dry (glove) boxes (1,3). These are used in academe where workers cannot afford the cost and storage of large stainless steel-type apparatus (4). After working with numerous water- and airsensitive materials, we found it imperative to construct a box of this sort. Herein we describe a modern approach to the fabrication of an inexpensive yet effective dry-box. Historically, inexpensive dry-box construction has incorporated the chemist's creativity as well as practicality, as demonstrated by Weyland and Schwartz (1). They utilized a discarded soda-pop cooler and fit it with a Plexiglas face. Bunce (2) built a box using plywood coated with epoxy varnish, and Shore (3) kept it simple by assembling a polyethylene bag over a rectangular framework. Our box consists of a silicone-bonded acrylic frame, PVC-pipe fittings, draw hasps, and polyethylene gloves. The UCSC Econo-Box has numerous advantages over its predecessors. First, its rigid acrylic design provides easy visualization and manipulation of objects. Second, its removable top and large chamber allow for the installation of analytical and top-loading balances. Third, its disposable polyethylene gloves not only maximize cleanliness and minimize contamination, but are also available in bulk at low cost. Finally, the system can readily be purged with inert gas to provide the appropriate atmosphere. Of course, the main advantage is the cost: $200. Table 1 compares the prices of commercially available dry-boxes. We have managed to build a box comparable in performance to ScienceWare's budget-priced glove box for 10% of their cost. 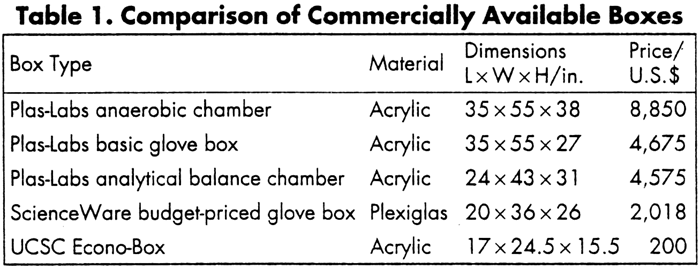
Table 2 lists our materials and their cost. Note that everything except the gloves (available from Fisher) and needle valves (available from a welding shop or industrial tool shop) was purchased at the local hardware store. 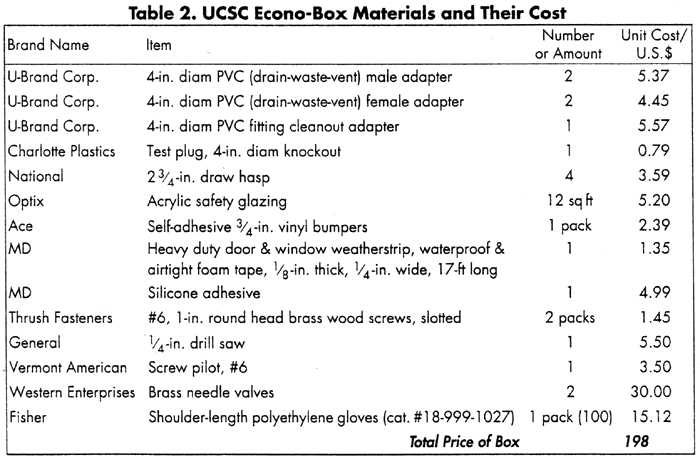
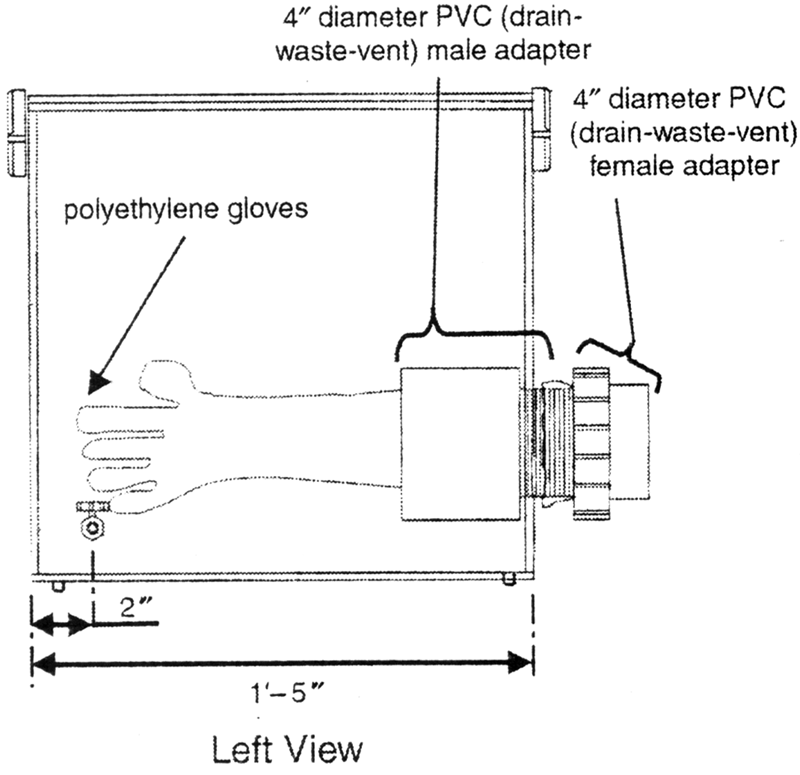
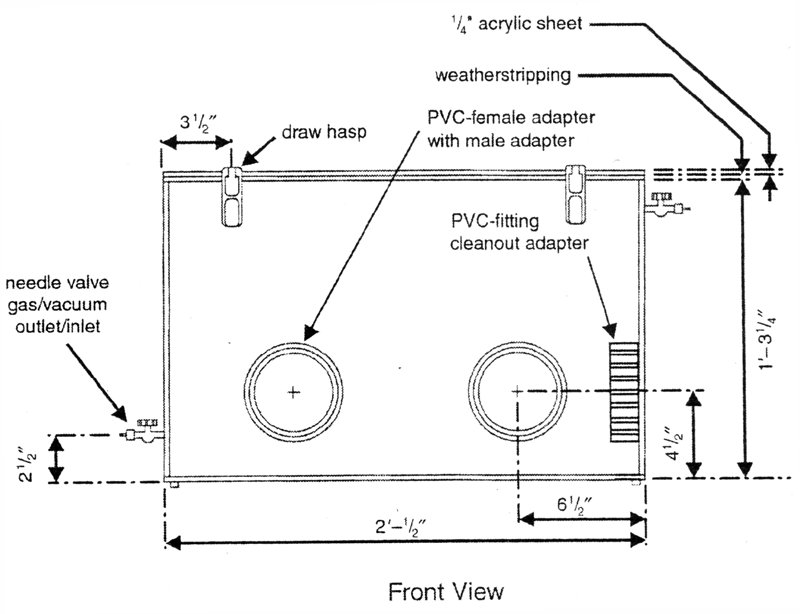
Construction of the box is as follows (see Fig. 1).
Methodology Evaluation Two experiments were performed to determine the oxygen and water content within the box. First, the box was purged with inert gas (argon or nitrogen) for one hour. Two trays were each charged with 20 g of Drierite containing indicator. One tray was placed inside the box and the other outside the box. After one hour, the sample within the box remained blue, while the sample outside the box became entirely pink, indicating the adsorption of water. The sample within the box required 72 hours to turn pink. In the second experiment, the oxygen content was determined using the reagent prescribed by Sekutowski et al. (5). First, the box was purged with argon for one hour. A dark blue solution of [Cp2TiClCl]ZnCl2*2THF was then prepared and syringed onto a glass plate within the dry-box. After 10 min, during evaporation of the THF, the solution became olive green, indicating >5 ppm of oxygen. After 20 min the solution became deep orange, indicating a >20 ppm oxygen content. Since the moisture test indicated the absence of water for more than three days whereas the oxygen test indicated the presence of oxygen within 20 min, the gloves must be semipermeable to oxygen but impermeable to water. Therefore, an improvement in this box would be to incorporate neoprene or equivalent dry-box gloves. These range in price from $100 to $200. Daily Use As an organic chemistry lab, we utilize materials such as acyl chlorides and boronate esters. Oftentimes it is necessary to isolate these compounds to obtain percent yield and characterization data. However, since they are highly sensitive to moisture and can be hydrolyzed in open air, their clean isolation can be a challenge. Thus, our main intent in designing a dry box was to make possible the isolation of moisture- and air-sensitive compounds. After using the box for 12 months, we present the best methodology for its use for this purpose. First, the gloves are cut and attached to the PVC fittings. A watch glass containing 20 g of Drierite is then placed within the box through the PVC-porthole. The moisture-sensitive material (e.g., a powder in a round-bottom flask sealed with a septum) is also transferred to the box along with any other necessary items. Vacuum grease is applied to the adapter and the porthole is sealed. If necessary, the removable top panel is also properly attached and sealed. At this point there should be no leaks or exposure to outside air. To purge the box, the needle valve, attached to house vacuum, is slowly opened and the inert gas valve is opened simultaneously. The rate of inert gas insertion is then adjusted to be greater than the rate of evacuation, so that the gloves are expelled, balloon-like, to the exterior of the box. This allows for removal of unwanted air as well as introduction of inert gas. It is recommended that the optimum rates be determined by observation of the box itself. For example, it will-begin to collapse if the vacuum is too high and expand if the vacuum is too low. The positive and negative pressures need not be extreme: our house vacuum typically pulls down to 200 Tarr and our inert gas pressure does not exceed 10 psi. After purging for 30 min, the moisturesensitive material is manipulated appropriately. Conclusions The UCSC Econo-Box has proved to be an effective tool in the handling of hygroscopic and air-sensitive materials. Its readily available and inexpensive components make its construction and assembly possible in one day, and its size renders it an alternative to the large industrial-Type boxes. For us, this box has provided a comfortable, relatively inert, moisturefree atmosphere-and at a low cost. For academe, that is most often the bottom line. Thus, it is our hope that you find it equally useful. 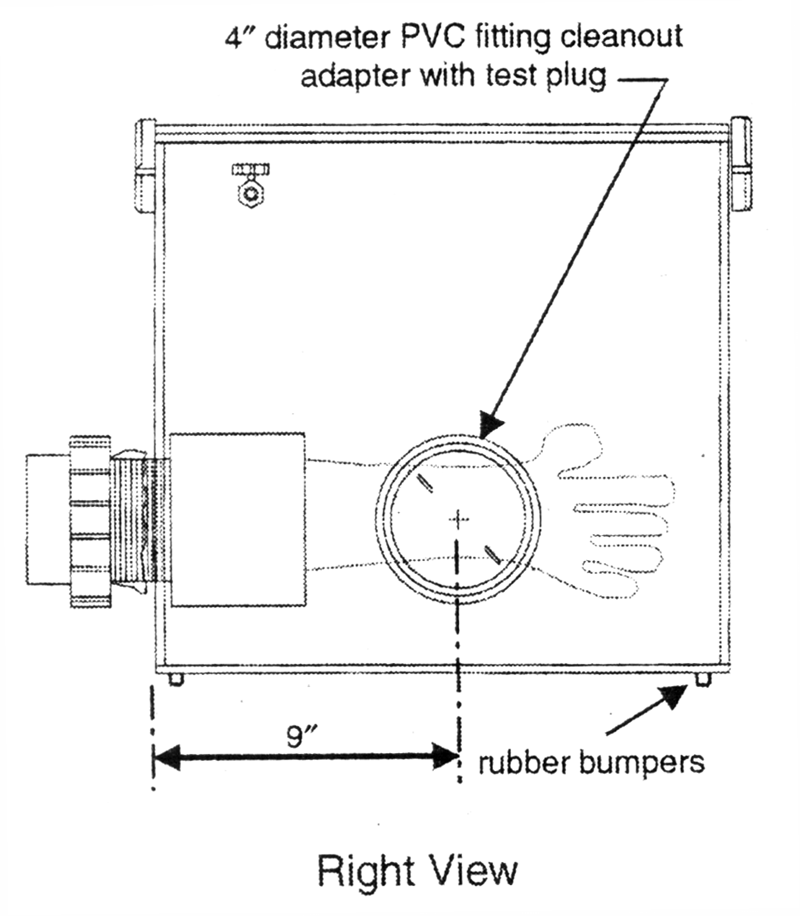
References [1] Weyland, H. H.; Schwartz, D. J. J. Chem. Educ. 1960, 37, 536.
|