I. Introduction
A. Background
The benzophenone/sodium still is a widely used method to produce water, oxygen, and peroxide free solvents for organic synthesis1,2. Most organic labs routinely use this method to dry their solvents that are used in moisture sensitive reactions. The most popular solvent for these reactions is tetrahydrofuran. However, despite widespread use there is no information available in the literature on how dry or oxygen free the solvent really is from this method. At Mallinckrodt Baker R&D we have developed a process to produce Ultra dry THF (less than 10 ppm water and peroxides), a study was initiated to compare our new material with material produced from the benzophenone/sodium still.
B. Benzophenone/Sodium Still
A literature search on the benzophenone/sodium still found only one reference to date on the subject1. The reference was from Chemical and Engineering News in 19781. The procedure in the article describes adding 5 grams of sodium, and 30 grams of benzophenone to 3 liters of tetrahydrofuran1. The mixture is then refluxed under inert gas until the blue color of the benzophenone ketyl forms. The author of the article states that "once the blue color forms, distillation of the solvent will yield very pure, dry, oxygen and hydroperoxide free ether"1. However, the author provides no data on the purity or water level of the solvent produced from this method. We decided to carry out a study on the benzophenone/sodium still to obtain these answers.
II. Results and Discussion
A. Benzophenone/Ketyl THF Still Purity Study
We set up a THF still following the exact procedure as in Chemical and Engineering News reference1. Analysis of the THF produced from the still gave 10.1 ppm of water with no dissolved oxygen or hydroperoxides. The GC analysis of the tetrahydrofuran from the still found that it contained 150 ppm of benzene as an impurity. A study was initiated to determine the source of the benzene. We tested both the tetrahydrofuran, and benzophenone starting material and found no benzene present. This indicated that the source of the benzene was from the chemical breakdown of the benzophenone in the still. A GC/MS analysis of the THF sodium/benzophenone mixture was carried out to help determine the possible mechanism of benzene formation. The GC/MS analysis showed compounds such as 1, 2, 3, and 4.
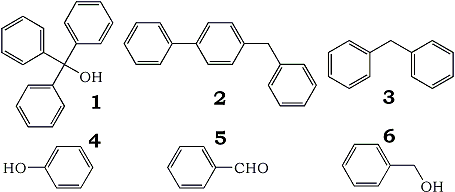
These compounds are formed by some type of free radical reaction with phenyl radicals. The probable mechanism is the carbonyl phenyl group bond breaks forming phenyl radicals, and benzaldehyde. The benzaldehyde (5) then either undergoes reduction to benzyl alcohol (6), or further reaction with the phenyl free radicals.
We carried out a test to determine if the benzaldehyde is reduced in the sodium/benzophenone still. Benzaldehyde (5) was added to THF in the presence of sodium, the mixture was then refluxed. We found that benzaldehyde (5) was reduced to the benzyl alcohol (6). Additional further GC/MS analysis was carried out to look for the benzaldehyde (5) and benzyl alcohol (6). Unfortunately, neither benzyl alcohol nor benzaldehyde was detected. One possible explanation is the benzaldehyde and benzyl alcohol rapidly undergo further reactions in the sodium/benzophenone still to prevent their detection. For example, the reaction of benzaldehyde with phenyl radicals could have resulted in the formation of compounds (1), and (2). Another possible pathway would be rapid polymerization of these compounds.
B. Effect of Water on the Benzophenone Ketyl
The next study was to determine what effect water had on the decomposition of the benzophenone ketyl. The benzophenone ketyl was produced in THF at a concentration of 630 ppm. The loss of blue color would indicate decomposition of the ketyl. Titration of the ketyl with water showed that a full 100 mol% of water was required to quench the blue color. This indicates that water reacts in a simple one to one mole ratio with the ketyl. Therefore, the blue color of the ketyl does not necessary indicate super dry conditions.
For example in the typical procedure the ketyl is produced at a concentration of 6311 ppm in THF. When a small amount of water is added to a sample of this mixture to increase the water concentration, the solution will still remain blue since until all of the ketyl decomposed. Thus it would be possible to have a concentration of 100 ppm water in the solvent, and if the ketyl was not used up, it would still have the blue ketyl color.
C. Effect of Oxygen on Benzophenone Ketyl
The final study was to determine how sensitive the ketyl was to oxygen. An experiment was carried out where the ketyl was titrated with oxygen. We found that the color was quenched with 8 mole% of oxygen. This data shows that the benzophenone ketyl reacts catalytically with oxygen and not on a one to one mole ratio as water does. Thus the benzophenone ketyl is much more sensitive to oxygen than to water.
II. Conclusion
Drying and purification of THF and other solvents is a widely used practice in many labs. Solvent produced from the sodium/benzophenone still is believed to be of the highest quality. Despite the fact there was no real data on the actual quality of the solvent. This belief has been passed down from lab worker to lab worker as the method of choice for producing anhydrous solvents for organometallic synthesis. Our studies have shown that while the THF produced from the still is free of oxygen and low in water (10 ppm), it does contain benzene contamination. The presence of benzene could interfere with critical applications. Additionally, the blue color of the benzophenone ketyl is not a very reliable indication of anhydrous conditions. These results are very significant since they provide the chemist real information on the purity of the tetrahydrofuran produced from the sodium/benzophenone still. In contrast our new THF is oxygen free, contains less that 5 ppm of peroxides. Our water content is less than 10 ppm and our material is free of impurities such as benzene.