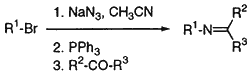Abstract
Secondary amines are obtained in moderate-good yields by reduction of crude imines prepared from N-alkyltriethoxyiminophosphoranes and aldehydes via the aza-Wittig reaction. N-Alkyltriethoxyiminophosphoranes are synthesized by one-pot azidation of alkyl bromides followed by Staudinger reaction.
Significant interest has developed in recent years in the application of iminophosphoranes for various synthetically useful transformations, especially those affording C=N bond possessing compounds, which are usually referred to as aza-Wittig reactions1. Although numerous articles have appeared on the reactions and synthetic applications of N-alkyl(aryl)triphenyliminophosphoranes2, the preparative utility of analogous aza-ylides, viz. N-allyltriethoxyiminophosphoranes is almost unexplored3. Recently, we reported the one-pot preparation of N-alkyltriethoxyiminophosphoranes from alkyl bromides by azidation followed by Staudinger reaction4 and the application of these compounds for the synthesis of primary amines5. Herein we describe a route for the synthesis of secondary amines beginning with the parent alkyl bromides and employing a facile construction of the final carbon skeleton via the aza-Wittig reaction between N-alkyltriethoxyiminophosphoranes and aldehydes. Secondary amines are synthesized by the variety of methods, the reduction of imines being a very convenient and explicit route6. Difficulties which may be sometimes encountered in this approach are due to the properties of some imines which are unstable and therefore not easily accessible in pure state. We decided to overcome this disadvantage by preparing the respective imines under mild conditions and use them for reduction without isolation and purification. The synthetic protocol illustrating the construction of secondary amine skeleton from two simple building blocks - alkyl bromide and aldehyde is presented in the Scheme.
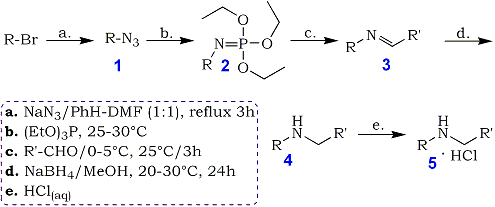
Azidation of alkyl bromides was performed for 3 hours at the reflux temperature of the solvent system, using a 100% excess of sodium azide. Benzene solution of crude alkyl azides 1 afforded N-alkyl- triethoxyiminophosphoranes 2 when treated with triethyl phosphite at 25-30°C.
Isolation and purification of these aza-Wittig reagents was neither necessary nor recommended. Neat 2 are extremely sensitive to traces of moisture and hydrolyze easily to diethyl N-alkylphosphoramidates which may then contaminate the target compounds. Benzene solution of 2 reacted easily with aliphatic and aromatic aldehydes to give the imines 3. If one followed the course of this reaction by 31P-NMR, the practically complete conversion of 2 to 3 was observed after 3 hours at room temperature. Due to the exothermic nature of this reaction, all reactions were started at 0-5°C and then run at 20-25°C.
Table 1.
Preparation of secondary amine hydrochlorides 5
| Entry | R | R' | Yield* | mp (solvent) |
| 1 | PhCH2CH2 | Ph | 77% | 259.5-261°C (95% EtOH) |
| 2 | C6H13 | Ph | 80% | 214-215°C (acetone) |
| 3 | CH2=CH-CH2 | Ph | 85% | 138-139°C |
| 4 | PhCH2CH2 | i-Pr | 75.5% | 239-240°C |
| 5 | C6H13 | i-Pr | 62% | 236-238°C (dec.) |
| 6 | PhCH2CH2 | Pr | 40% | 215-218°C (dec.) |
| 7 | C6H13 | Pr | 32% | 248-250°C (dec.) |
| 8 | sec-Bu | Pr | 19% | 196-197°C |
* Overall yield of crude 5 calculated on alkyl bromide
The crude imines 3 were not isolated but immediately reduced with sodium borohydride in methanol to form the target secondary amines 4. The amines were purified by steam distillation and characterized as corresponding hydrochlorides 5. This methodology can be used to prepare a number of secondary amines as shown in Table 1. The range of substrates in Table 1 demonstrates the clear utility of this synthetic procedure in preparing a variety of secondary amines in moderate to good yields. Best results were obtained when benzaldehyde and primary alkyl bromides were used as starting materials. The overall yields of 5 were inevitably lower (entry 6 and 7) with easily enolizable butyraldehyde as carbonyl substrate, possibly due to base-promoted (by strongly basic 2) aldol condensation as a side reaction.
Although secondary alkyl bromides could be easily converted into the corresponding iminophosphoranes 24, the aza-Wittig reactions of the latter were totally ineffective (entry 8).
Experimental
General
Unless otherwise stated, all solvents and reagents were purchased from commercial suppliers and used without further purification. Aldehydes were freshly distilled before use. Melting points were determined in open capillaries and are uncorrected. All new compounds gave satisfactory elemental analyses data. Infrared spectra were measured using a Specord M80 (C. Zeiss) instrument, and NMR spectra were recorded at 80 MHz with a Tesla BS 587FT spectrometer. All amine hydrochlorides exhibited IR and NMR spectra in accord with the expected structures.
Preparation of Secondary Amine Hydrochlorides 5; General Procedure:
A suspension of finely powdered sodium azide (6.5 g, 0.1 mol) in the mixture of alkyl bromide (0.05 mol), benzene (15 mL), and dimethylformamide (15 mL) was refluxed gently with stirring for 3 h. In the case of allyl bromide azidation should be carried out at 35-40°C for 6 h. The product was cooled to room temperature and poured into cold water (200 mL). The organic layer was separated. The aqueous layer was extracted with benzene (3x15 mL); the extracts were combined with the organic phase, dried (MgSO4), and filtered. Triethyl phosphite (8.3 g, 0.05 mol) was added dropwise with stirring to a benzene solution of crude alkyl azide 1. The temperature of the slightly exothermic reaction was kept at 25-30°C for 4 h and the product was left overnight at room temperature. Benzene solution of N-alkyltriethoxyiminophosphorane 2 such prepared was added dropwise with stirring and occasional external cooling (ice-water bath) to a solution of freshly distilled aldehyde (0.05 mol) in benzene (5 mL) at 0-5°C. After the addition had been completed the mixture was stirred for 3 h at 20-25°C. Benzene solution of crude imine 3 was then evaporated under reduced pressure end the residue was diluted with methanol (100 mL). Sodium borohydride (1.9 g, 0.05 mol) was added portion-se with stirring to the resultant solution and the temperature was kept below 30°C. The mixture was then left at room temperature far 24 h, evaporated under reduced pressure and diluted with water (50 mL). The solution was made strongly alkaline With an excess of 50% aqueous sodium hydroxide and steam distilled. The distillate was acidified with 25% hydrochloric avid and evaporated to dryness. Crude amine hydrochloride 5 was dried in vacuo over P2O5 and crystallized from the suitable solvent (see Table 1). When the amine could not be distilled with steam (entry 1) the crude reduction product after evaporation of methanol was steam distilled to remove volatile contaminants.
The residue was made alkaline and extracted with ether (3x50 ml). The extracts were evaporated, the residue was treated with 25% hydrochloric acid (10 mL), and evaporated to dryness to give crude 5. Analytically pure samples were obtained by recrystallization from ethanol-ether unless otherwise stated (see Table 1).