Abstract
A case in which aminorex (cis (±)-4,5-dihydro-5-phenyl-2-oxazolamine) was prepared as a "designer" analog of a controlled substance, methylaminorex (cis-(±)-4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine) is described. The confiscated drug sample was analyzed by high-field (300-MHz) proton nuclear magnetic resonance (NMR) and carbon-13 NMR spectroscopy, as well as electron impact mass spectrometry (70 eV). These examinations proved conclusively that the material in question was aminorex.
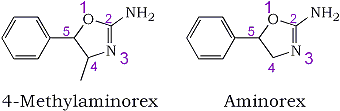
An illicit compound which has recently been identified in several solid samples seized in Florida and California is cis-(±)-4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine, also known as methylaminorex, ice, or euphoria (U4Euh)1. This oxazoline exerts potent central nervous system sympathomimetic effects not unlike those of methamphetamine2-4. Methylaminorex was first prepared in 1962 as a potential anorexic agent5. There appears to be widespread use of this designer drug, and illicit use of this compound has resulted in at least one fatality1. Through the efforts of various law enforcement agencies, this substance has recently been controlled (Schedule I) under emergency scheduling provisions of the controlled substances act6,7. With this scrutiny, alternatives to the methylaminorex structure have been sought to avoid potential prosecution. For this reason, the desmethyl derivative, that is, aminorex, was synthesized (see Fig. 1). It is an interesting circumstance that aminorex was reported in the same literature as was methylaminorex. Aminorex, under the trademark Menocil®, proceeded to the point that it was introduced into the European market (West Germany, Austria, and Switzerland) as an anorexic agent in February of 1966. During its use, an unacceptably high incidence of pulmonary hypertension was reported, which led to the deaths of several individuals8-10. These side effects caused this drug to be withdrawn from the market in November 1968. A new drug application (NDA) which was pending in the United States was also discontinued at that time. The illicit use of this formerly marketed drug as an alternative to its methylated analog was recently observed and is described here.
Materials and Methods
Chemicals
The compound cis(±)-4,5-dihydro-4-methyl-5-phenyl-2-oxazolamine, which was used for comparative purposes, was prepared according to the method of Poos et al.11. All the solvents used were of reagent or spectroscopic quality and were obtained from Aldrich Chemical Co.
Analytical Procedures
The melting points were determined on a Thomas-Hoover capillary melting-point apparatus and were uncorrected. Proton (1H) and carbon-13 (13C) nuclear magnetic resonance (NMR) spectra were obtained using a Vanan XL 300 spectrometer. The samples were dissolved in an appropriate deuterated solvent, usually deuterated chloroform (CDCl3), and chemical shifts8 were reported relative to an internal standard [tetramethylsilane (TMS)]. The mass spectra were recorded on a Kratos MS8ORFA Spectrometer. Thin-layer chromatography utilized EM reagent DC-aluminum foil plates, coated to a thickness of 0.2 mm with silica gel 60.
Results and Discussion
A sample of a white powder was recently confiscated from a white male in Florida. The suspect indicated that the seized material was not a controlled substance. The solid was analyzed by various analytical methods, including high- field (300-MHz) proton (1H) and 13C NMR spectroscopy and electron impact (EI) mass spectrometry (70 eV). ln addition, the purity of the solid was determined chromatographically. The melting point of the sample was found to be 124 to 127°C, which was lower than the reported melting point for either methylaminorex (150 to 152°C) or aminorex (136 to 138°C)11. Thin-layer chromatographic analysis revealed an Rf value for the material in question of 0.43 when eluted on silica plates using a mobile phase containing 85:10:5 ethyl acetate/methanol/concentrated ammonium hydroxide. Several impurities were detected at Rf values both greater and less than that of the compound of interest. Another indication of the impurity of the samples examined was their microcombustion profiles, which indicated deviation from the accepted carbon values of between 2 and 15%.
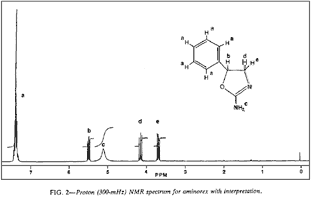
Fig 2. [Enlarge]
Aminorex 1H NMR Spectrum with Interpretation.
The proton NMR spectrum for aminorex and its structural assignments are provided in Fig. 2. Unlike the spectrum of methylaminorex, no upfield (<3 δ) absorbances characteristic of a methyl group occur in the 1H NMR spectrum of the confiscated sample.
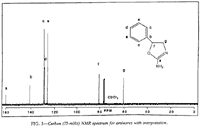
Fig 3. [Enlarge]
Aminorex 13C NMR Spectrum with Interpretation.
The obtained spectrum is indicative of the assigned structure of aminorex, with the phenyl group appearing at 7.35 δ, a benzylic methine triplet at 5.46 δ, the exchangeable amino group at 5.2 δ, and the nonequivalent hydrogens of the methylene group β to the phenyl ring at 4.13 and 3.65δ. The 13C NMR spectrum is equally supportive of the 4,5-dihydro-5-phenyl-2-oxazolamine structure, with no upfield methyl signal and with the remaining carbon absorbances occurring at their expected 8 values (Fig. 3). Decoupling analysis of this spectrum indicates quaternary and secondary carbons at 160.89 δ, 140.62 δ, and 60.63 δ, corresponding to the imine carbon (C-2), the phenyl carbon attached to C-5, and the methylene carbons (C-4). The remaining methine carbons manifested signals at 128.62 δ, 128.14 δ, 125.66 δ, corresponding to the phenyl carbons, and at 81.43 δ, which was assigned as the C-5 absorbance.
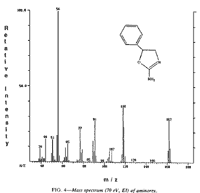
Fig 4. [Enlarge]
Aminorex EI/MS Spectrum.
Mass spectrometric analysis was performed on the sample in question by probe distillation introduction. This technique indicated the presence of impurities which vaporized at higher temperatures than did the object compound. An El spectrum of the material is given in Fig. 4. The fragmentation pattern and the molecular ion are indicative of the aminorex structure and compare well with those of standard spectra12. The M+ peak is 162 m/z. Loss of the carboxamide group CONH2 produces the fragment at 118 m/z, while generation of protonated benzyl alcohol results in the m/z = 107 fragment. Other important fragments include the tropylium ion (91 m/z) and the phenyl cation at 77 m/z. The base peak of the spectra (56 m/z) appears to be due to the imine structure, NH2-C=N-CH2+. The mass spectrum reported for methylaminorex is characterized by an M+ of 176 m/z and a base peak of 70 m/z, each differing by 14 mass units from the corresponding aminorex fragments. The collected analytical information leaves little doubt that the compound in question was the desmethyl derivative, aminorex, of the controlled substance methylaminorex.
Conclusions
A case was identified in which a drug formerly marketed as an anorexic agent, aminorex, was found to be used in an apparently illicit manner as a substitute for methylaminorex. Analysis of a recently impounded sample conclusively proved that the material was aminorex, an identification based on 1H and 13C spectroscopy and mass spectrometry.