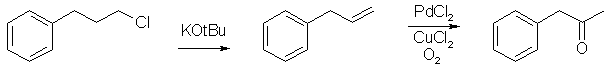
Synthesis of P2P from 3-phenyl-1-chloropropaneby KrZ[ Back to the Chemistry Archive ] DehydrohalogenationBegin by purchasing 1kg of 3-Phenyl-1-chloropropane AKA 3-phenylpropyl chloride. This should easily be had for $180 or less. It is unwatched and while many of your suppliers might not have it in stock, they can all source it for you. Next get yourself 800g of Potassium tertbutoxide ($140 per kg or less). Now all we need is some tBuOH, go ahead and get 4L (Not more than $75). Place the 1Kg of 3-phenylpropyl chloride, 800g Potassium tertbutoxide, and 2L of tBuOH in a 5L RBF and stir well for 24-48hours. Slight heating (not refluxing) might help, or then again it might hurt. This is all that has been tested so far. When the 24 hous is up filter off the KCl which will fall out of solution during the reaction. Take your remainder and distill off your tBuOH, vacuum distill and collect a large allylbenzene fraction (BP will depend on your vacuum of course). As soon as the allylbenzene stops coming over, shut it down. You’ll be left with a small brown-yellow mess in the flask and 645g of Allylbenzene or about an 85% yield. OxidationPlace the 645g of Allylbenzene in a 4L Rossi wine jug, along with 3L of anhydrous EtOH, 6.5g PdCl2, 20g CuCl2*2H2O, and 50ml of distilled water. By balancing a stir bar on the dimple in the middle you can get it spinning nicely. Now firmly attach an O2 input hose to the top of the jug (see other posts for details). Pressurize the device to 45psi (numerous times this has been attempted and none of them have resulted in the jug exploding) and stir vigorously for 8 hours. The cleanup for this reaction has already been covered far to extensively elsewhere (spiceboys writeup on rhodium for example), so I won’t get into it. Once you have completed your extraction and distillation you will be left with 660g of very fun to play with P2P. You can either take this and perform NaBH4 reduction or Al/Hg or H2 or whatever your little heart desires. Some additional notes
|