Synthesis of Adrafinil and ModafinilUS Pat 4,066,686 & 4,177,290Benzhydrylsulphinyl-acetohydroxamic Acid (Adrafinil)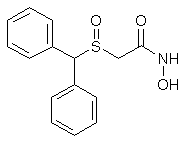
Diphenylmethanethiol15.2 g (0.2 mol) of thiourea and 150 ml of demineralized water are introduced into a 500 ml three-neck flask equipped with a central mechanical stirrer, and with a dropping funnel and a condenser on the (respective) side-necks.The temperature of the reaction mixture is brought to 50°and 49.4g (0.2 mol) of bromodiphenyl- methane are added all at once whilst continuing the heating. After refluxing for about 5 minutes, the solution, which has become limpid, is cooled to 20°C and 200 ml of 2.5 N NaOH are then added dropwise whilst maintaining the said temperature. The temperature is then again kept at the reflex for 30 minutes after which, when the mixture has returned to ordinary temperature (15-25°C), the aqueous solution is acidified with 45 ml of concentrated hydrochloric acid. The supernatant oil is extracted with 250 ml of diethyl ether and the organic phase is washed with 4x80 ml of water and then dried over magnesium sulphate. 39 g of crude diphenylmethane-thiol are thus obtained. Yield 97.5%. Benzhydryl-thioacetic acid10 g (0.05 mol) of diphenylmethane-thiol and 2g (0.05 mol) of NaOH dissolved in 60 ml of demineralised water are introduced successively into a 250 ml flask equipped with a magnetic stirrer and a reflux condenser. The reactants are left in contact for 10 minutes whilst stirring, and a solution consisting of 7g (0.075 mol) of chloroacetic acid, 3g (0.075 mol) of NaOH pellets and 60 ml of demineralized water is then added all at once. The aqueous solution is gently warmed to about 50°C for 15 minutes, washed with 50 ml of ether, decanted and acidified with concentrated hydrochloric acid. after filtration, 10.2g of benzhydryl-thioacetic acid are thus obtained. Melting point 129-130°C. Yield 79%. Ethyl benzhydryl-thioacetateThe following reaction mixture is heated under reflux for 7 hours: 10.2 g (0.0395 mol) of benzhydryl-thioacetic acid, 100 ml of anhydrous ethanol and 2 ml of sulphuric acid. When heating has been completed, the ethanol isevaporated in vacuo; the oily residue is taken up in 100 ml of ethyl ether and the organic solution is then washed with water, with an aqueous sodium carbonate solution and then with water until the wash waters have a neutral pH. After drying over sodium sulphate, the solvent is evaporated. 10.5g of ethyl benzhydryl- thioacetate are thus obtained. Yield 93%. Benzhydryl-thioacetohydroxamic acidThe following three solutions are prepared:
The solutions are heated, if necessary, until they become limpid, and when the temperatures have again fallen to below 40°C, the solution of potassium hydroxide in methanol is poured into the solution of hydroxylamine hydrochloride in alcohol. Finally, at a temperature of about 5° to 10°C, the solution of ethyl benzhydryl- thioacetate is added in its turn. After leaving the reactants in contact for 10 minutes, the sodium chloride is filtered off the limpid solution obtained is kept for about 15 hours at ordinary temperature. The methanol is then evaporated under reduced pressure, the residual oil is taken up in 100 ml of water and the aqueous solution is acidified with 3 N hydrochloric acid. The hydroxamic acid which has crystallized is filtered off, washed with water and then dried. 9.1 g of product are obtained. Yield = 87.5%. Melting point 118-120°C. Adrafinil (CRL 40,028)10.4g (0.038 mol) of benzhydryl-thioacetohydroxamic acid are oxidized at 40°C, over the course of 2 hours, by means of 3.8 ml (0.038 mol) of hydrogen peroxide of 110 volumes strength (33%), in 100 ml of acetic acid. When the oxidation has ended, the acetic acid is evaporated under reduced pressure and the residual oil is taken up in 60 ml of ethyl acetate. The product which has crystallized is filtered off and then purified by recrystallisation from a 3:2 (by volume) mixture of ethyl acetate and isopropyl alcohol. 8 g of Adrafinil are thus obtained. Melting point 159-160°C. Yield 73%. Solubility in water <1 g/litre. Benzhydrylsulphinylacetamide (Modafinil)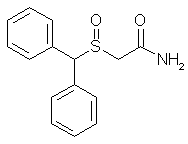
Benzhydrylthioacetyl chloride19.5g (0.076 mol) of benzhydrylthioacetic acid in 114 ml of benzene are placed in a three-necked flask provided with a condenser and a dropping funnel. The mixture is heated and 19 ml of thionyl chloride are added drop by drop. Once the addition is complete, the reflux is continued for about I hour, cooling and filtering are carried out and the benzene and the excess thionyl chloride and then evaporated. In this way, a clear orange oil is obtained. Benzhydrylthioacetamide35 ml of ammonia in 40 ml of water are introduced into a three-necked flask provided with a condenser and a dropping funnel and the benzhydrylthioacetyl chloride dissolved in about 100 ml of methylene chloride is added drop by drop. Once the addition is complete, the organic phase is washed with a dilute solution of soda and dried over Na2SO4, the solvent is evaporated and the residue is taken up in diisopropyl ether; in this way, the benzhydrylthioacetamide is crystallized. 16.8 g of product (yield 86%) are obtained. M.p. 110°C. Modafinil (CRL 40,476)14.39 g (0.056) of benzhydrylthioacetamide are placed in a balloon flask and 60 ml of acetic acid and 5.6 ml of H2O2 (about 110 volumes, 33%) are added. The mixture is left in contact for one night at 40°C. and about 200 ml of water are then added; the CRL 40476 crystallizes. By recrystallization from methanol, 11.2 g of benzhydrylsulphinylacetamide are obtained. Yield: 73%. M.p. 164-66°C. |