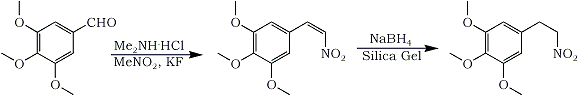
3,4,5-Trimethoxy-beta-nitrostyrene
The following components were placed in a one-necked 1000ml conical flask equipped with a Dean-Stark water trap (capacity about 30ml): 3,4,5-trimethoxybenzaldehyde (98.1 g, 0.5 mol), dimethylammonium chloride (81.5g, 1 mol), nitromethane (300ml), toluene (300ml) and anhydrous potassium fluoride (4.36 g, 75 mmol). This mixture was vigorously refluxed with stirring for 5 h. The reaction flask was cooled down, then fitted to a rotary evaporator to order to remove the volatiles by gradual heating under reduced pressure. To the tepid residue (~55°C), chloroform (125 ml) and 0.2 M HCl (400 ml) were added. The mixture was heated on the water bath until complete dissolution. Then the flask was stored overnight in a refrigerator (-5°C). A crystalline solid was filtered out by suction, carefully rinsed with water and thoroughly dried in a vacuum oven. The filtrate was poured into a separatory funnel, the layers were separated and the aqueous phase was extracted with chloroform (3x100 ml). The organic extracts were combined then evaporated to give a crude oily material, which was chromatographed over silica gel (400g, eluent dichloromethane-ethyl acetate, 95:5). After removal of the solvent, the resulting solid and the previously separated product were recrystallized together from isopropanol: yield 99.2g (83%); mp 125.5-126.5°C (allotropic change at 121-121.5°C).
3,4,5-Trimethoxyphenyl-2-nitroethane
The following components were placed in a 6000ml wide-mouthed reaction flask provided with an efficient mechanical stirrer: isopropanol (750 ml), chloroform (2500ml) and the above nitrostyrene (47.8 g, 0.2mol). When the crystals had dissolved completely, silica gel 200-400 mesh ASTM (400g) was poured into the flask whilst the mixture was continuously stirred vigorously. Sodium borohydride (33.25 g, 0.88 mol) was then added portionwise over a period of 15 min. The slurry was stirred for an additional 2 h, and acetic acid (~50 ml) was carefully added. The insoluble material was separated by suction and the filtrate was evaporated in vacuo (the recovered solvents were used to rinse the silica gel thoroughly). The resulting crude material was taken up with dichloromethane (500 ml) and water (300 ml). The organic layer was separated, and the aqueous phase was extracted with dichloromethane (3x100ml). The combined extracts were dried with magnesium sulfate, filtered, then evaporated to dryness. The residue was chromatographed on a silica gel column (400g, eluent dichloromethane-ethyl acetate, 95:5) to give 3,4,5-Trimethoxyphenyl-2-nitroethane (46g), which was further purified by recrystallization (benzene-cyclohexane) to yield 44.6g (92.5%) as colorless crystals, mp 82-83°C.