2C-H via 2,5-Dimethoxymandelonitrileby KrZThis spectacularly successful new method comes to us from J. Org. Chem. 1982, 47, p. 2638-2643. In this article the authors first attempted to produce 2-methoxy-PEA from the nitrostyrene, however, when they attempted to scale-up this reaction they encountered a number of persistent bugs, in their own words the reaction was "fickle" when scaled up. Although nitrostyrene synthesis is relatively easy, it's reluctance to dissolve in a useful hydrogenation solvent has led to many problems. Using GAA and H2SO4 as a dimer inhibitor is the preferred method, but yields are still not as spectacular as one might hope, and it takes 2L of GAA to dissolve just 100g of nitrostyrene. For these reasons we have arrived at the present method, a relatively easy, very high yielding, and very scalabe reaction for the production of 2CH and Mescaline. So let's get started. Reagents
Procedure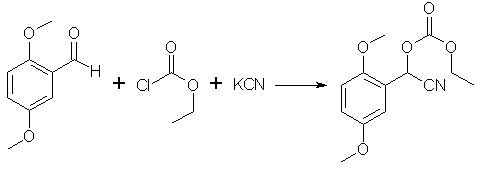
250g of 2,5-Dimethoxybenzaldehyde [1.5 mol] is dissolved in 600ml of THF, 162 g of Ethyl Chloroformate [1.5 mol] is added and the solution is stirred in an ice bath until cool. 98g of KCN dissolved in 250ml of water is added all at once, the mixture is kept at 0C for 8hrs. After this period of time the ice is allowed to melt, and the reaction is allowed to warm up to room temperature for an additional 10hrs. Upon completion, 1500ml of water is added, and the mixture is extracted 3x with 125ml of DCM. The combined DCM extracts are dried with sodium sulfate, filtered, and the residue is distilled under heavy vacuum to afford 356.8g of the mandelonitrile as an pale yellow, creamy, thick liquid [bp 115-120°C @ 0.3 mmHg], yield 83.5%. 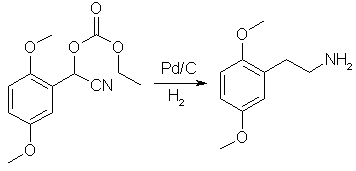
The entire mass of mandelonitrile is dissolved in 2L of EtOH containing 175g of H2SO4 and 20g of 10% Pd/C are added. Eiter a high-pressure hydrogenation or an atmospheric pressure hydrogenation. The only difference between the two will be the reaction time, at 80psi 100% absorption occurs after 90 minutes, at atmospheric, a 90% yield will be obtained by bubbling the EtOH with a stream of H2 for a period of 8 hours [Or use a balloon, but don't pull a beaker]. When the hydrogenation is complete the reaction mix is filtered to recover the Pd/C which can be washed with additional MeOH and reused several times. The filtrate is the cooled on an ice bath and 71.5g of NaOH is slowly added with stirring, upon completion, if done slowly enough, the mixture will still be cool. As the NaOH is added you will not the precipitation of a large mass of Sodium Sulfate, when done, allow the mixture to stir on the ice bath for another 30 minutes to assure complete precipitation of the Sodium Sulfate. The sodium sulfate is filtered off, and the reaction mix goes directly to distillation where 205.88g of 2CH will be recover, a 90% yield. Additional NotesIf doing a high-psi hydrogenation, the solvent quantity can be cut in half, and possibly more, making this reaction immensely versatile. When applied to 3,4,5-trimethoxybenzaldehyde, the yield of mandelonitrile will be cut to ~80%. I hope this will satisfy those people who have gotten on my case before, claiming that my write-ups use nothing but high-psi techniques. Alpha-beta: A while back during the discussion of Krz's KCN ethylchloroformate reaction to make 2-CH, people wanted one with easier to get reagents. Another person mentioned the reaction of KCN with the bisulfite addition product of 2,5-Dimethoxybenzaldehyde and NaHSO3. Well I did some digging and found this gold mine in a german reference. I don't speak german, but here is the translation anyway, If any german speaking bee's would be willing to translate the paragraph PM me and I'll email you a jpg of the page. Methoxy-substituted Mandelonitriles from their corresponding Benzaldehydes: To a solution of 40g NaHSO3 (sodium bisulfite) in 360ml water heated to 40°C, 44 g 4-Methoxybenzaldehyde (0.324 mol) is added with vigorous stirring. After 20 min, cool the reaction mixture down to 0°C, add 200 mL diethyl ether and add a solution of 17 g NaCN in 70 mL water dropwise. Let stir for 30 min at 0°C, separate the organic phase and extract the aqueous phase twice with 50 mL portions of ether. The combined ether extracts are then dried over anhydrous calcium chloride. After the evaporation of the solvent under a vacuum at 35-40°C, the remaining oil is cooled and allowed to solidify. The entire mass of crystals is sucked dry on a buchner funnel and is pure enough for hydrogenation. Looks good eh? Now just slap this in a Hydrogenation flask with Pd/C, EtOH and H2SO4, and blammo, enough 2-CH to kill a horse. Important: My question is, will the mandelonitrile reduce to the PEA w/ Pd/C at 1 atm pressure or will it reduce to the amino alcohol. At higher pressure I'm certain that the benzylic alcohol would be removed, but at one atm I'm not so sure. Krz's mandelonitrile route doesn't actually synth the mandelonitrile, it synthesizes the O-ethoxycarbonyl ester, which would be much easier to reduce IMHO. Rhodium: Archiv der Pharmazie 283, 190 (1950) 0.1 mol of aldehyde in ether is treated with 0.125 mole of ethyl chloroformate and a solution of 9.8g of KCN and 31g MgSO4*7H2O in 50ml water. The mixture is stirred vigorously with cooling in ice for 3h. After that the etheral layer is separated, and washed with water and then with 10% Na2CO3 until no more chloride could be detected in the washings. The etheral solution was washed with 30% NaHSO3, and then with water to remove the excess sulfite. After thorough drying over CaCl2 and evaporating the ether, the O-carbethoxymandelonitrile is gotten in a good yield, pure enough for the next step. |