Hydroquinone Diacetate1
Procedure
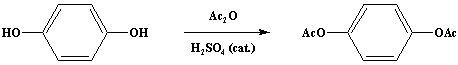
One drop of concentrated sulfuric acid is added to a mixture of 110 g. (1.0 mole) of hydroquinone and 206 g. (190.3 ml., 2.02 moles) of acetic anhydride (Note 1) in a 1 L Erlenmeyer flask. The mixture is stirred gently by hand; it warms up very rapidly, and the hydroquinone dissolves. After 5 minutes the clear solution is poured onto about 800 ml. of crushed ice. The white crystalline solid which separates is collected on a Buchner filter and washed with 1 L of water. The filter cake is pressed occasionally to facilitate the removal of water; the solid is dried to constant weight over phosphorus pentoxide in a vacuum desiccator. The nearly pure product weighs 186-190 g. (96-98%) and melts at 121-122°C (Note 2); it can be recrystallized from dilute ethanol (Note 3).
Notes
- The use of commercial acetic anhydride in this preparation sometimes results in appreciably lower yields. The checkers used the freshly redistilled reagent.
- The melting point is recorded in the literature as 121°C and as 123-124°C.
- Recrystallization from 50% ethanol (by weight) permits a 93-94% recovery of material melting at 121.5-122.5°C; about 365 g. (400 ml.) of the solvent is required for 100 g. of the crude product.
2,5-Dihydroxyacetophenone2
Procedure
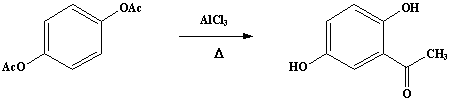
A mixture of 50g (0.257 mole) of dry hydroquinone diacetate and 116g (0.87 mole) (Note 1) of anhydrous aluminum chloride is finely powdered in a mortar and introduced into a dry 500-mL RB flask fitted with an air condenser protected by a calcium chloride tube and connected to a gas-absorption trap. The flask is placed in an oil bath (Note 2) which is heated slowly from room temperature so that at the end of about 30 minutes the temperature of the oil reaches 110-120°C, at which point the evolution of hydrogen chloride begins. The temperature is then raised slowly to 160-165°C and maintained at that point for about 3 hours (Note 3); at the end of about 2 hours the evolution of hydrogen chloride becomes very slow and the mass assumes a green color and becomes pasty in consistency (Note 4).
The flask is removed from the oil bath and allowed to cool to room temperature. The excess aluminum chloride is decomposed by treating the reaction mixture with 350g of crushed ice followed by 25mL of concentrated hydrochloric acid. The solid obtained is collected on a Büchner funnel and washed with two 100mL portions of cold water. The crude product weighs about 35g (89-90%). Recrystallization from 4L of water yields 25-30g (64-77%) of green, silky needles melting at 202-203°C (Note 5).
Notes
- Ordinary commercial aluminum chloride can be used. If the amount of this reagent is less than 3 moles per mole of the ester the yield diminishes. To compensate for any inert ingredients in the commercial aluminum chloride, the reagent is employed in an excess of about 10% over 3 moles.
- The flask should not touch the bottom of the oil bath; if it does, the lower portion of the mixture may char.
- If the evolution of the hydrogen chloride becomes vigorous, the calcium chloride tube may be removed temporarily and the top of the condenser connected directly to the gas-absorption trap. When the gas evolution slackens and there is no longer any danger that the calcium chloride tube will be blown off, the guard tube is reinserted.
- The reaction requires about 2 hours, but the heating is continued another hour to ensure its completion.
- The product may be recrystallized from 250mL of 95% ethanol rather than from the much larger quantity of water.
2,5-Dimethoxyacetophenone3
Coming soon...