Amine |
Imine Formation Time |
NaBH4 Ratioa |
Imine Reduction Time |
Yieldb |
| EtNH2 | 1 h |
0.5 |
1 h |
52% |
| EtNH2 | 2 h |
0.5 |
1 h |
55% |
| EtNH2 | 1 h |
1.0 |
1 h |
56% |
| EtNH2 | 2 h |
1.0 |
3 h |
73% |
| MeNH2 | 1 h |
0.5 |
1 h |
55% |
| MeNH2 | 2 h |
0.5 |
1 h |
56% |
| MeNH2 | 1 h |
1.0 |
1 h |
49% |
| MeNH2 | 2 h |
1.0 |
3 h |
78% |
|
||||
Freifelder stated a long time ago that phenylacetone and aqueous methylamine formed N-methylimine which is hydrogenated to N-methylamphetamine. The wet enviroment doesn't disturb the imine formation. Yet when bees here want to make MDMA they tend to choose anhydrous conditions, in particular in the sodium borohydride reduction of the imine gotten from MDP2P and methylamine.
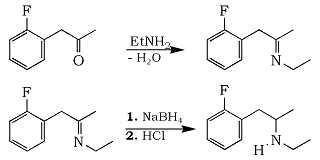
I decided to challenge this, and performed a series of experiments using the legal substrate 1-(2-Fluorophenyl)-2-propanone, with varying reaction conditions.
Variables explored were the ratio between NaBH4 and ketone, different alkylamines and reaction time for imine formation and imine reduction, respectively. The results can be seen in the table, and the procedure used for all experiments is found below.
Procedure
1-(2-Fluorophenyl)-2-propanone (10g, 65mmol) was dissolved in 50ml toluene and to this was added a solution made of Ethylamine HCl (10.6g, 130mmol) and NaOH (5.25g, 130 mmol) in 50ml water. The mixture was vigorously stirred at room temp for 2 hours. The aqueous phase was then removed and the toluene phase was transferred to a 250ml RB flask containing 1.9g NaBH4 (50mmol), 25ml water and 15ml 95% EtOH and the mixture was vigorously stirred for a further 2 hours at room temp. Dilute hydrochloric acid was then added dropwise until pH 2 was reached, the phases was separated and the toluene phase was extracted twice with 20ml 5% HCl and then discarded. The combined aqueous phases was made strongly alkaline with 50% aqueous NaOH and extracted twice with 50ml toluene. The toluene extracts was dried over MgSO4 and stripped of solvent in a rotovap. The residual yellow oil (nasty smell) was dissolved in 50ml EtOAc and dry HCl in IPA added until pH 4 was reached. The white crystals was isolated and dried to constant weight.
Yield: 8.9g (40.9 mmol, 62.9%) of N-Ethyl-1-(2-Fluorophenyl)-2-aminopropane hydrochloride.