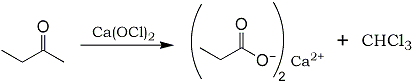Hypochlorite Oxidation of Methyl Ethyl Ketone
The oxidation of a methyl ketone with hypochlorite (OCl-) results in the formation of a carboxylic acid salt and
chloroform, and is a special case of the so-called haloform reaction. The general equation for this type of synthesis is:
2 RCOCH3 + 6 Ca(OCl)2 -> (RCOO)2Ca + 3 CaCl2 + 2 Ca(OH)2 + 2 CHCl3 (in this case, R represents the ethyl radical, or C2H5-).
Calcium Hypochlorite Oxidation of Methyl Ethyl Ketone
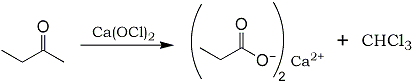
Using methyl ethyl ketone (MEK) as the ketone and either calcium hypochlorite (bleaching powder) or sodium
hypochlorite (Chlorox), we can get propionic acid and chloroform as products. With the procedure below, using calcium
hypochlorite, the yield of chloroform is ~45% and that of propionic acid is ~40%, calculated on methyl ethyl ketone.
Experimental1
300 grams of Calcium Hypochlorite (commercial bleaching powder, 24% available chlorine) was made into a
paste with 750 mL of water at 15°C. The temperature of the water must be above 10°C, otherwise too viscous a
paste results. This mixture was put into a 3-liter flask which was equipped with a dropping funnel, mercury-
sealed stirrer and condenser. Then 25 mL of methyl ethyl ketone was gradually introduced with stirring, care
being taken to avoid frothing over. The mixture became quite warm and 10 mL of chloroform, a 45% yield,
distilled. The refractive index of the distillate was 1.4452 (lit. 1.4458).
The residue in the flask was neutralized with nitric acid. Then more nitric acid was added to liberate the
propionic acid, purposely adding less than the calculated amount to avoid subsequent extraction of nitric acid.
Even with an excess, however, ether extracts only insignificant quantities of nitric acid from a dilute aqueous solution.
To determine the exact yield of propionic acid, continue as in the section below. To isolate the acid, extract it
from the aqueous layer with with several portions of diethyl ether. If another solvent is used, such as chloroform or
methylene chloride, be careful as to not add an excess of nitric acid, as it has a considerable solubility in chlorinated
solvents. On a larger scale, the propionic acid can also be isolated by distilling its azeotrope with water, followed by
solvent extraction of the distillate, to minimize the volumes handled
The propionic acid can be purified by precipitating it as its sodium salt, placing it in a distillation flask and
liberate it by the slow addition of sulfuric acid.
Analysis of the formed Propionic Acid
The acidified solution was diluted to 2000 mL with water. An aliquot portion of 500 mL of this was
extracted with 4x50 mL ether for the organic acid and the extracts diluted to 250 mL with ether.
Titration of an aliquot portion of 50 mL of this ether solution with 0.2016 N alkali required 27.5 mL.
This corresponds to a yield of 8.18 g or 40.7% of the calculated amount of propionic acid.
Nitric Acid Oxidation of n-Propanol2
Concentrated (70%) Nitric acid (710g, 500 mL) and 50 mL of water were introduced into a vessel equipped with a mechanical stirrer.
n-Propanol (144.5g, 180 mL) were slowly added over a period of two hours, while the contents of the vessel were stirred
vigorously and cooled to maintain the temperature at 30-35°C. A reflux condenser was attached to the vessel so as to
prevent the loss of low-boiling products while allowing exhaust fumes, consisting primarily of nitrogen oxides (toxic and irritating!) and carbon
dioxide, to escape. Propionic acid was obtained in 82.5% yield and acetic acid in 10.8% yield. The conversion of the
n-propanol was 100%.
The reaction products can be separated by distilling off the azeotropic mixture of the aliphatic acid and water from
which the acid can be recovered by solvent extraction, e.g. with a mixture of benzene and ethyl acetate.
Important: Be sure to read the entire original publication2 before trying this synthesis out. It contains essential
information about the evolution of nitric oxide gas from the reaction mixture, as well as the need for cooling,
especially after the initial induction period before the exothermic oxidation gets going.
Hydriodic Acid Reduction of Lactic Acid3
The formation of propionic acid by the action of hydriodic acid on lactic acid, orignally observed by Lauteman, has been confirmed by the author, who finds, moreover that this reaction affords a convenient mehtod for the preparation of propinoic acid.
670 g Iodine is suspended in 140 mL water, and converted to hydriodic acid by means of hydrogen sulfide, and the hydriodic
acid thus obtained is placed in a distillation setup with 60 g of lactic acid, after which the mixture is distilled until about 100g of
liquid have passed over. A reflux condenser being now adapted to the apparatus, and the contents of the flask are digested for
about four hours, when the iodine, which is by this time has crystallised in the condenser, is washed back into the
flask with the 100g distillate previously drawn off, and hydrogen sulfide is passed through the mixture in order to reduce
the free iodine. The precipitated sulfur being removed, 100g are again distilled off, the contents of the flask are digested
during another period of four hours, and this series of operation repeated six or seven times, the whole of the lactic acid
being converted into propionic acid, almost the whole of which is contained the 100g drawn off after the last digestion.
This distillate is now mixed with 50 mL of water, and distilled as long as the contents of the receiver contain only traces
of hydriodic acid; the distillate is then neutralized with sodium carbonate, and sufficient liquid propionate is added to
convert the sodium iodide into sodium propionate, The solution is then evaporated, and the dry sodium propionate is
decomposed with hydrochloric acid gas, as directed by Linneman4. Lactic acid when treated in
this way yields about 62% of pure sodium propinate.