Introduction
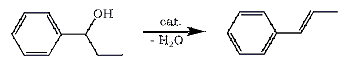
Phenyl-1-propanol (P-1-Pol) is a benzylic alcohol, easily prepared by reduction of propiophenone1,5, or by the alkylation of benzaldehyde with either EtMgBr2, Et2Zn (60%)3 or Et2Zn/CeCl3 93%)4.
| Compound | Phenyl-1-Propanol | Propenylbenzene |
| Formula | C9H12O | C9H10 |
| Mol. Wt. | 136.19 g/mol | 118.18 g/mol |
| Boiling point | 211-212°C 97-98°C/10mmHg 106-108°C/18mmHg |
176-177°C 66-67°C/14mmHg 76-78°C/19mmHg |
| Density | 1.00 g/mL (20°C) | 0.91 g/mL (20°C) |
Dehydration of Phenyl-1-propanol with any suitable catalyst like p-Toluenesulfonic Acid or Potassium Hydrogen Sulfate will eliminate water from the alcohol, resulting in the formation of a high yield (80%+) of propenylbenzene (a.k.a. β-Methylstyrene or 1-phenylpropene), which is an excellent precursor for the synthesis of phenyl-2-propanone, (meth)amphetamine, cathinones as well as ephedrine alkaloids.
This general procedure is not limited to the preparation of solely unsubstituted propenylbenzenes, as it has successfully been used in the past for other analogs5.
Dehydration of Phenyl-1-propanol to Propenylbenzene
Using p-Toluenesulfonic Acid
50.3g (0.369 mol) redistilled Phenyl-1-propanol (P-1-Pol), 1.002g p-Toluenesulfonic acid and approx 150ml Toluene was placed in a 500 mL two-necked RBF with stir bar and thermometer in one neck just to see the vapor temperature, then up for reflux with a Dean-Stark trap.
Oil bath was set to 130°C and reflux rate was less than 2 drops/sec. Reflux slowed when 6.2ml of H2O was collected. Vapor temperature climbed from 113°C to 116°C and oil bath climbed to 140°C with reflux starting again, washing the rest of the water droplets from the condenser and surrounding area into the trap. Heat was stopped and a total of 6.5ml H2O was collected. This took about 4 hours.
The solution was transferred to a 500ml separation funnel and washed with 65ml 10% NaHCO3 then 65ml Brine. The solution was a clear light yellow, and this was poured into a 400ml beaker with stir bar and dried with some CaCl2, then filtered into a 500ml RBF and set up for fractional distillation with a Claisen adaptor packed with Raschig rings. The oil bath was heated to 70°C and the toluene was removed by vacuum distillation.
The residue was cooled and transferred to a 100ml RBF and the 500ml flask was washed with a little toluene, which was added to the solution in the small RBF. The 100ml RBF was heated at atm pressure to collect the last of the Toluene, then at 174°C-175°C came the propenylbenzene (Oil bath was at 210°C). When nothing more distilled over at that temp, vacuum was applied to get the last drops over, resulting in a total recovery of 34.2g propenylbenzene (78.4% yield) and in the distillation flask 6.7g of a brown viscous mushy liquid remained, which upon testing was found to be acid to litmus.
Using Potassium Hydrogen Sulfate
55g Phenyl-1-propanol, with all DCM removed, was placed in a 250ml RBF with stir bar, and 1.2g KHSO4 was added and set up for simple distillation using a recovery tube instead of a distillation head.
With oil bath at 147°C, small bubbles were seen. At 158°C, water began forming in the recovery tube and falling back into the flask causing fizzing and white smoke. This problem was effectively curtailed by the use of a heat gun aimed at the recovery tube every time water appeared.
At about 183°C, the water droplets started to appear oily. At 198°C no more water was seen to evolve and propenylbenzene started coming over (along with water), but no more need for the heat gun. Over 205-210°C, the benzene was coming over fast, then slowed.
The oil bath was taken up to 220°C when the rest of the benzene appeared to come over. After that, the temp was taken to 228°C when a light yellow liquid appeared and distillation was stopped. 56.7g total was collected and 2.8g remained in the reaction flask.
Transferred to a 250ml sep funnel, ~6.1g water was separated and the propenylbenzene was drained into a 250ml Erlenmeyer flask, the receiver flask and sep funnel was washed with 10ml DCM, which was then combined with the propenylbenzene.
Added some CaCl2, left for an hour and filtered into a 100ml RBF with stir bar. Washed the Erlenmeyer flask and filter cake with 3x20ml DCM and added these washes to the propenylbenzene as well.
Set up for fractional vacuum distillation, the DCM evaporated at a bath temp of 50°C, and then at a temp of 54°C, the product distilled over at 42-43°C/5.5 mbar to give crystal clear propenylbenzene in a yield of 83.8%. Yeah!
Better than I've ever done and I feel sure I can get over 90%. I have a feeling that water falling back into the reaction flask lowers the yield and some white stuff can be seen in the condenser and recovery tube when this happens. Using a distillation head and thermometer will guarantee the water falling back in, even with a heat gun. Heat tape is a probably an even better idea.