Abstract
An efficient and short synthesis of [1-amino-2-(3-indolyl)-ethyl]-phosphonic acid (1), the phosphonic acid analog of tryptophan, from indole-3-acetic acid which utilizes a novel zinc-copper couple reduction of an oxime is described.
The phosphorous analogs of α-amino acids have been studied extensively due to the finding that these class of molecules often have interesting biological activities ranging from plant growth regulators1 to antibacterial agents2. In addition, the incorporation of these molecules into small peptides have produced potent inhibitors of a variety of enzymes of medicinal importance3. Thus, the 1-aminophosphonic acids represents a useful and interesting series of compounds.
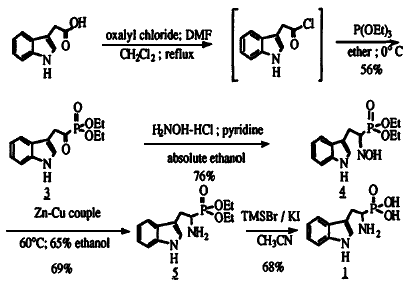
Scheme 1
Several good methods for the synthesis of these compounds have been reported. For instance, the diaryl esters of a variety of amino acid analogs can be prepared by a two step synthesis from benzyl carbamate, aldehyde, and triphenylphosphine4 or via aminomethylphosphonate schiff base intermediates5. In addition, oxidation of the corresponding phosphinic acids have also been reported6. However, noticeably absent from the literature are reports of an efficient synthesis of the phosphonic acid analog of tryptophan 1. The single reported synthesis of 1 proceeds in poor yield (10%), requires the isolation of reactive indole-3-acetyl chloride 2 and suffers a cumbersome Al-Hg reduction of oxime 47. Thus, we wish to report a more efficient synthesis of j which eliminates the need for the isolation of 1 and relies on a novel Zn-Cu reduction of 4 (Scheme 1). While our synthesis follows that of Subotkowski et. al., in general, it is important to note that our improved method provides higher overall yields (20%) and begins with commercially available indole-3-acetic acid.
Experimental
Indole-3-acetyl chloride 2, was generated from indole-3-acetic acid using oxalyl chloride (1.1 eq.) in refluxing methylene chloride which contained 0.3% DMF by volume. After a solvent exchange to anhydrous diethyl ether the acid chloride was reacted with triethyl phosphite (1 eq.) at room temperature to generate the Arbuzov product, keto-phosphonate (3) which precipitates out of ether as a white solid in 56% yield. This compound exists primarily in the enolate form, as identified by 1H-NMR. The keto-phosphonate was converted to the oxime (4) upon treatment with hydroxylamine hydrochloride (1.3 eq.) and pyridine (1.5 eq.) in absolute ethanol at room temperature. The ethanol was removed and the reaction was treated with 1 N HCl and extracted with CH2Cl2. The organic layer was cooled to 0°C which caused 4 to precipitate out as a white solid in 76% yield. Initially, the reduction of the oxime to the amine was performed with an aluminum amalgam7. However, the poor yields (20-40%) of the reaction and the large quantities of mercury waste generated indicated that a better route was needed. Hydrogenation over Raney-Nickel proved ineffective. It was found that the reduction of the oxime to the racemic amine 5 could be accomplished in good yield (69%) using zinc-copper couple in warm aqueous ethanol with ultrasonic agitation8. This novel method for the reduction of oximes is vastly preferable to the aluminum-amalgam reductions previously reported. Finally, the cleavage of the phosphonate esters to form 1 was accomplished in 68% yield with TMSI, generated in situ by the reaction of TMSBr and KI in acetonitrile9.
In summary, tryptophan phosphonic acid was synthesized in five steps from indole-3-acetic acid. This procedure has advantages over the previously published route in that the critical acid chloride is generated in situ and the Al-Hg reduction was replaced with a more efficient zinc-copper couple method. These advantages make this scheme amenable to production of large quantities of 1.