Ultrasound Promoted Nitrostyrene SynthesisTet. Lett. 39, 8013-8016 (1998)[ Back to the Chemistry Archive ] The Knoevenagel condensation reaction of benzaldehydes with nitroalkanes is a classic general method for the preparation of nitroalkenes, which are very valuable synthetic intermediates. A multitude of promoters have been developed for these reactions including acids, bases and ammonium salts. Typical conditions to effect the condensation of nitroalkanes with aromatic aldehydes consist of heating an acetic acid solution of the aldehyde with the appropriate nitroalkane with ammonium acetate at 100°C for a few hours. In this way nitroalkenes 5 may be isolated in 30-95% yield depending on the aldehyde used. We have observed lower yields of nitroalkenes result from the condensation of electron-rich aromatic aldehydes, a result that may be general. For example, under these standard Henry conditions, 2,3-dimethoxybenzaldehyde 1a condensed with nitromethane to give the nitroalkene 5a in only 35% yield, the mother liquors being contaminated with resinous material we attributed phenol-formaldehyde type polymerization. Recent reports concerning the ultrasound promoted carbonyl addition reactions led us to consider the application of ultrasound as a low temperature promoter of the above Henry reaction. 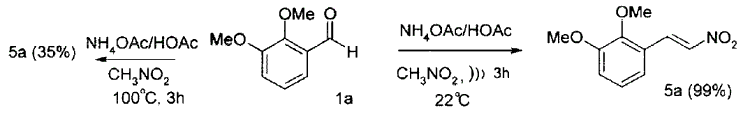
Repetition of the reaction of 1a with the same reagents but at room temperature and application of ultrasound led to a rapid, clean condensation (complete in 3h) and subsequent isolation of the nitroalkene product 5a in 99% yield, with no resinous side products being produced. This result prompted us to investigate the scope of the method further. General proceduresNitromethane and pyridine (Aldrich) were freshly distilled. Ultrasound reactions were performed using a Branson 5510 ultrasound bath or a Crest Tru-Sweep ultrasonic cleaner with little difference. Method A: A mixture of aldehyde (20.0 mmol), nitromethane (13.0 mL), glacial acetic acid (3.3mL) and ammonium acetate (3.324g) was sonicated at 22°C for 3h. After removal of nitromethane, partition between dichloromethane and water then brine gave crude product which was recrystallized from aq, ethanol (except 5d, AcOH). Method B: A solution of aldehyde (1.0 mmol), nitromethane (1.0 mL), ammonium acetate (2.5 mmol) and diisopropylethylamine (0.1 mmol) was sonicated at 22°C for 3-6h, according to TLC. After removal of solvents, work-up as above gave the crude product, which was purified on silica gel. Table 1: Starting benzaldehyde, Yield (Method)
No reaction occurs under these conditions at room temperature until ultrasound is applied. In addition, attempts to conduct the ultrasound promoted reaction at room temperature without acetic acid or without ammonium acetate, failed. Other studies using primary or secondary amines in place of ammonium acetate were not as successful. The ultrasound promoted reaction proved to be general for a variety of electron rich aromatic aldehydes under these conditions. High yields of nitroalkenes were isolated for all alkoxy-substituted aromatic aldehydes investigated. Thus, piperonal 1b reacted to give nitroalkene 5b (99%) while the 2,4,6-trimethoxy derivative 1c gave nitroalkene 5e (84%) demonstrating steric factors to be of little detriment. The hetero-substituted aldehydes 1d and 1e and indole-3-carboxaldehyde 1f also produced the corresponding nitroalkenes efficiently, as did vanillin 1g without protection of the phenolic hydroxyl. Electron deficient aromatic aldehydes also reacted under our ultrasound promoted conditions but the major product proved to be the corresponding nitroaldols, isolated in poor yield. Modification to the conditions by changing to base catalysis improved the yields to 50-70%. Sonication of a solution of 4-chlorobenzaldehyde 1h according to method B allowed for isolation of the nitroaldol 4h in 70% yield. This method proved general for electron deficient aldehydes. Both 2-nitro- and 4-nitrobenzaldehyde gave the corresponding nitroaldols 4i and 4j in good isolated yield. |