Of the two required isomeric aldehydes, 2,5-dimethoxy-3-methylbenzaldehyde (I) and 2,5-dimethoxy-4-methylbenzaldehyde (II), the latter was synthesised from 2,5-dimethoxytoluene by a Gattermann reaction; but this and several other direct methods failed to convert 4-methoxy-2-methylphenol (III) into the corresponding aldehyde. Compound (I) was finally prepared by oxidation with selenium dioxide1 of 3-hydroxymethyl-2,5-dimethoxytoluene (IV), which was synthesised by two different procedures.
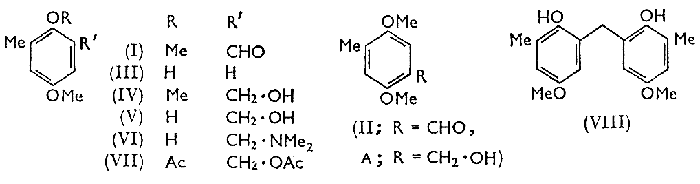
By the Lederer-Manasse reaction2, with formaldehyde and calcium hydroxide in water, 4-methoxy-2-methylphenol (III) was converted into 2-hydroxymethyl-4-methoxy-6-methylphenol (V) which gave compound (IV) on methylation.
The alcohol (V) partially decomposed during distillation under reduced pressure to an uncharacterised phenolic material. From the reaction of o-toluquinol and formaldehyde followed by methylation, Euler and his co-workers3 isolated a compound (A) to which they assigned the structure (IV) on the basis of its conversion into a 2,5-dihydroxymethylbenzoic acid which depressed the melting point of 2,5-dihydroxy-4-methylbenzoic acid prepared by the Kolbe reaction. The compound (A) was not found by us to be the same as our product (IV) and we have identified it as 4-hydroxymethyl-2,5-dimethoxytoluene by obtaining the aldehyde (II) from its oxidation with selenium dioxide, and 2,5-dimethoxy-4-methylbenzoic acid4 by alternative oxidation procedures. By this result the structural assignment of the aldehyde (II) is also confirmed.
The second synthesis of compound (IV) was of poor yield. It involved a Mannich reaction of compound (III) with formaldehyde and dimethylamine5 yielding 2-dimethylaminomethyl-4-methoxy-6-methylphenol (VI), which, when heated with acetic anhydride6, gave 2-acetoxy-5-methoxy-3-methylbenzyl acetate (VII). This was hydrolysed with alkali and compound (IV) was obtained by methylation of the hydrolysate. A by-product of this reaction was bis-(2-hydroxy-5-methoxy-3-methylphenyl)methane (VIII), which may have arisen from the reversible decomposition of 2-hydroxymethyl-4-methoxy-6-methylphenol (V) to give 4-methoxy-2-methylphenol (III) and formaldehyde, followed by the condensation of compound (III) with compound (V) or with an intermediate arising from compound (V) by loss of a molecule of water.
Experimental
2,5-Dimethoxy-4-methylbenzaldehyde (II)
(a) This compound was synthesised in 89.6% yield from 2,5-dimethoxytoluene, hydrogen cyanide, and aluminium chloride, according to the standard Gattermann procedure7, it had bp 111-116°C/0.9mmHg, mp 85-86°C.
(b) To compound (A)3 (18.2 g.), mp 74.5-75°C, dissolved in benzene (60 ml), finely powdered selenium dioxide (5.5g) was added and the suspension was azeotropically distilled until the formation of water stopped. Distillation of the residue under reduced pressure gave 2,5-dimethoxy-4-methylbenzaldehyde (11.5g, 63.8%), mp 84-85°C, having an infrared spectrum identical with that of the material obtained in (a), and causing no depression of its melting point.
2-Hydroxymethyl-4-methoxy-6-methylphenol (V)
Calcium oxide (28 g) was added gradually to a suspension of 4-methoxy-2-methylphenol (138g) in water (800 ml) and 37% formalin (81 g) under nitrogen. The mixture was shaken until solution was complete and then kept overnight. The solution solidified when shaked; it was diluted with water and acidified with acetic acid, and the oily phenol was extracted with ether. The extract, after being washed with sodium hydrogen carbonate solution and water and dried (Na2SO4), was evaporated. Distillation of the product (137.5g) under reduced pressure caused extensive decomposition. The distillate of 2-hydroxymethyl-4-methoxy-6-methylphenol, bp 136-140°C/1mmHg, had mp 58-59°C after crystallisation from isohexane.
3-Hydroxymethyl-2,5-dimethoxytoluene (IV)
(a) To a solution of crude compound (V) (137.5g) in acetone (200 ml) were added anhydrous potassium carbonate (115 g) and methyl iodide (125 g). The solution boiled, and boiling under reflux was continued for 18 h, then the solution was poured into a large volume of water, and the precipitated oil extracted with ether. The extract was washed with 2N sodium hydroxide solution and repeatedly with water, then dried (Na2SO4) and distilled in a vacuum to give a main fraction, bp 109-116°C/0.6mmHg, nD24 1.5327-15344, of slightly impure 3-hydroxymethyl-2,5-dimethoxytoluene [98.2 g, 54% based on (III)] .
(b) To a mixture of compound (III) (20 g) and 23% dimethylamine (29g) was added 37% formalin (13g) dropwise with stirring and cooling to keep the temperature below 20°C. After it had been stirred for 2 hr. the mixture was extracted with benzene and the extract dried (Na2SO4) and evaporated to give crude 2-dimethylaminomethyl-4-methoxy-6-phenol (VI) which was heated under reflux with boiling acetic anhydride (50g). This solution was then diluted with water and extracted with benzene, the extract washed with water, sodium carbonate, and then water, the benzene was evaporated, and the residue (VII) hydrolysed in boiling aqueous methanolic sodium hydroxide for 3h. This alkaline solution was acidified and extracted with ether. From this extract impure (VIII), mp 126-130°C, was obtained. The alkaline solution containing compound (V), was methylated (dimethyl sulphate), acidified with hydrochloric acid, and extracted with ether. On evaporation of the extract-two fractions were obtained, (i) bp 113-118°C/1mmHg, nD22.5 1.5350 (3.1g), having an infrared spectrum identical with that of the material prepared in (a) ; (ii) bp 205°C/0.9mmHg, mp 135-137°C after crystallisation from n-hexane (4.2 g.). The latter was identified as bis-(2-hydroxy-5-methoxy-3-methylphenyl)methane (VIII)
2,5-Dimethoxy-3-methyl benzaldehyde (I)
Finely powdered selenium dioxide (30g) was added to a solution of compound (IV) (95.8g) in benzene (100 ml), and the suspension azeotropically distilled until the formation of water was complete (3.5g of water collected). The residue was distilled under reduced pressure and after a fore-run of red oil (6.2g), 2,5-dimethoxy-3-methylbenzaldehyde (71.5g, 75%), bp 89-91°C/0.5mmHg, mp 40-41°C, was collected. It crystallised from ligroin as white needles, mp 42°C.