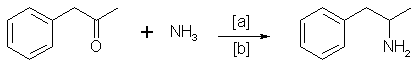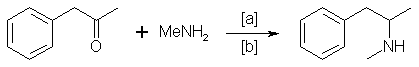Impurities in Illicit Drug Preparations: Amphetamine and Methamphetamine
Dr. Anthonie M.A. Verweij, Forensic Sci. Rev. 1(1), 1-11 (1989)
[ Back to the Chemistry Archive ]
Abstract: In this review, attention is paid to chromatographic and mass spectral properties of already
identified impurities found to be present in frequently abused drug preparations of illegal origin of amphetamine
and methamphetamine. The most commonly employed methods of synthesis of drugs of this type are briefly
described. Special emphasis is given to the Leuckart route, found to be the preferred method, in the illicit
production of amphetamine. Furthermore, some isolation and preconcentration methods for the contaminants
are discussed. The importance of identifying impurities present in amphetamine or methamphetamine cannot
be overestimated. These impurities originate mostly from the improper purification in the end stage of the
different syntheses used in the clandestine manufacture of the substances; it is possible to differentiate between
the several kinds of illegal drug preparations, synthesized by various methods, by means of so-called "route
specific" impurities. Finally, a survey is given of the impurities already known to be present in amphetamine and
methylamphetamine, together with their mass spectral and some chromatographic properties.
Introduction
A recurrent theme in forensic drug analysis concerns the possibilities of locating sources of supply and manufacture of illicit drugs by means of
diagnostic chemical or physical properties [17,38]. Powder formulated drugs and their potential accompanying substances can be examined only on the basis
of their chemical properties; whereas with tablets or capsules their visual appearance and the nature of bulking, binding, lubricating, diluting and
coloring agents can play an important role.
In contrast to genuine drugs, illegal drug preparations are often contaminated. Impurities in these preparations largely depend on inadequate purification
procedures, and they can originate from a variety of causes such as imperfect chemical handling, starting materials, side, and subsequent reactions,
intermediate products, diluents, laboratory dirt, and from handling and packing the drugs. It stands to reason that chromatographic patterns obtained from
illegally produced drugs might contain valuable information about the drug and its method of synthesis, even if the identity of only a limited number of
the chromatographic peaks is known. In general, only patterns are compared and, if possible, recognized in these so-called "chemical signature" analyses
[38]. In recent years some basic work has been done [23,35] regarding the nature of contaminations encountered in the different syntheses of amphetamine
or methamphetamine. Mostly it is related to the Leuckart reaction as the most popular method for the production of amphetamine in both the western European
countries and the U.S. [8,35].
In this review, the syntheses commonly used in the clandestine preparation of amphetamine and methamphetamine are discussed, followed by some remarks
on the isolation of impurities from reaction mixtures and final preparations. Lastly some chromatographic and mass spectrometric data of the various
impurities are given. The impurities present in amphetamine and methamphetamine are accentuated here, since the contaminants present can give important
indications about the type of synthesis used. A contribution has been made in improving the information content of the "signature" analysis of amphetamines.
Today even the presence of "reaction specific" impurities can be established in the chromatographic profiles.
Due to the scarcity of data, little attention can be given to inorganic impurities found in clandestine amphetamine or methamphetamine originating
from hydrogenating catalysts.
Synthesis of Amphetamine
Over the years the Leuckart reaction has remained the most popular method for synthesizing illicit amphetamine in the U.S. [8], the U.K. [32], and The
Netherlands. The reductive amination of benzyl methyl ketone is important [8,16], while in Sweden and the U.S., the nitropropene route is incidentally
used, like the phenyloxime route in the U.S. The following are some methods for the production of amphetamine, in which the chemical handling is not
difficult and the necessary materials are easy to purchase. Many other methods are known, but are of lesser importance in this context. For further
information refer to reference [32].
A. The Leuckart Rection
This reaction can be formulated by the following scheme:
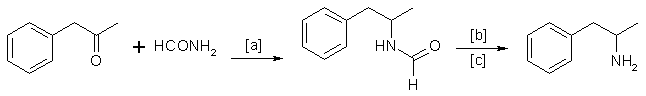
in which [a]: 180-190°C; [b]: H2SO4/HCl (dilute); [c]: 90-125 °C.
Reaction conditions can vary [29]. A trend [15] in recent years has been to replace formamide by ammonium formate [48] or a mixture of ammonia and formic acid [6].
B. The Reductive Amination of Benzyl Methyl Ketone
Benzyl methyl ketone can react with ammonia in the following way [13]:
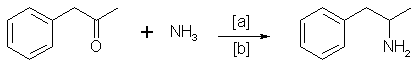
in which [a]: Raney Nickel, Pt, H2, Al powder in the presence of HgCl2, Nickel plated Zinc; [b]: 20-170°C, 1-130 atm, ethanol, methanol.
Reaction conditions can differ widely [1,5,11,36]. (Only low pressure and low temperature aminations have been encountered so far in the Netherlands.)
C. The Oxime Route
Benzyl methyl ketone reacts with hydroxylamine to give the oxime, which can be hydrogenated to give the amphetamine [14]:
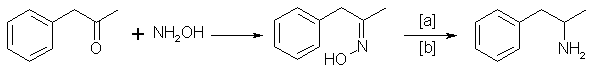
in which [a]: Na (amalgamated), Na (absolute ethanol), LiAlH4, or H2 and Raney Nickel, nickel, iron, nickel plated zinc; [b]: 20-170°C, 1-130 atm;
Electrolytical reduction has also been reported. Great differences have been described for the reaction conditions [18,27,31].
D. The Phenylnitropropene Route
Condensation of benzaldehyde with nitroethane yields 1-phenyl-2-nitropropene [2,9]. Hydrogenation of the double bond and subsequent reduction of the nitro group gives the amphetamine:
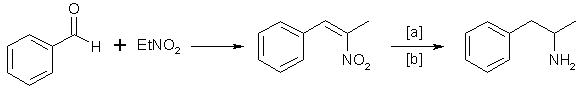
in which [a]: LiAlH4, H2 and Raney Nickel or Pd/C; [b]: 20-100°C, 1-80 atm, CH3OH, C2H5OH, H2O/HCOOH, C2H5OH.
Reaction conditions vary widely [4,10].
Synthesis of Methamphetamine
In contrast to the western European countries, in the U.S. the illicit production of methamphetamine has preference over clandestine amphetamine
production. (In The Netherlands, methamphetamine has rarely been produced up to the current time. Only once in the past 10 years has a high
pressure reductive amination of benzyl methyl ketone with methylamine been found [46], operating under near-professional standards.)
A. The Reductive Amination of Benzyl Methyl Ketone
Illicit methamphetamine is primarly produced in the U.S. [8] by reductive amination, according to the following scheme:
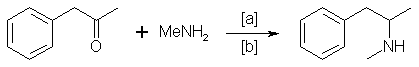
in which [a]: HgCl2/Al, NaBH4 in slightly acid medium, H2/Pd, Na/ethanol, H2, Raney Nickel; [b]: 25-160 °C, 1200 atm, methanol, ethanol, ethyl
ether. Reaction conditions vary widely.
B. The Leuckart Reaction
In methamphetamine preparation, the following reaction is of minor importance, compared with reductive amination. Schematically:
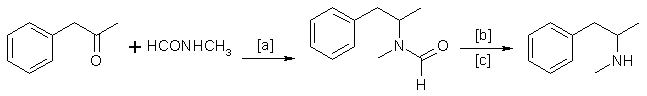
in which [a]: 170-190°C; [b]: H2SO4 or HCl; [c]: 120-170 °C. Instead of N-methylformamide, a mixture of methylamine and formic acid is sometimes
used [23].
Of far lesser importance are the syntheses, in which ephedrine is used as the starting material. Several routes using ephedrine have been reported,
including: (a) hydro genating ephedrine [12] in acidic solutions using Pd/ BaSO4 and H2 at elevated temperatures (100 °C); (b) reacting ephedrine with
chlorine (from thionyl chloride or phosphorus pentachloride) and subsequent hydrogenation of the intermediate [20] 1-phenyl-1-chloro-2- methylaminopropane;
(c) reducing of ephedrine with hydrogen iodide and red phosphorus [20].
A typical example of thoughtless chemical handling is the reaction in which benzylmagnesium chloride is refluxed with the condensation product
of methylamine and acetaldehyde. Due to imperfect chemical handling the efforts to produce methamphetamine were fruitless [8].
A wide variety of methamphetamine syntheses are known but of lesser importance. For further details, refer to references [3,32].
Survey of Impurities
The impurities reported in the literature are given here according to the sequence developed in the section (II) on the syntheses of amphetamine
and methamphetamine.
| Table 1. Impurities found in amphetamine synthesized with
the Leuckart reaction |
| Name |
Remarks |
Ref |
| Formamide |
a |
- |
| Formic Acid |
a |
24 |
| Phenylacetone |
a |
24 |
| N-formylamphetamine |
b |
24 |
| 4-Methyl-5-phenylpyrimidine |
c,d |
45 |
| 4-Benzylpyrimidine |
c,d |
45 |
| Dibenzyl ketone |
e |
24 |
| alpha-Benzylphenethylamine |
c |
22 |
| N,N-Di(beta-phenylisopropyl)amine |
c |
24 |
| 2,4-Dimethyl-3,5-diphenylpyridine |
c |
42 |
| 2,6-Dimethyl-3,5-diphenylpyridine |
c |
42 |
| 4-Methyl-5-phenyl-2-(phenylmethyl)-pyridine |
c |
42 |
| 2-Methyl-3-Phenyl-6-(phenylmethyl)-pyridine |
c |
42 |
| N,N-Di(beta-phenylisopropyl)methylamine |
c |
24 |
| 2,4-Dimethyl-3-phenyl-6-(phenylmethyl)-pyridine |
c |
44 |
| 2-Methyl-2-phenylmethyl-5-phenyl-2,3-dihydropyrid-4-one |
c |
43 |
| N,N-Di(beta-phenylisopropyl)formamide |
c |
23 |
| Table 2. Impurities found in amphetamine synthesized by
the oxime route and the phenylnitropropene route |
| Name |
Remarks |
Ref |
| 2-Phenyl-methylaziridine |
c |
21 |
| 2-Methyl-3-phenyl-aziridine |
c |
21 |
| Benzyl Methyl ketoxime |
b |
8 |
| Phenyl-2-nitropropene |
b,f |
8 |
| Table 3. Impurities found in amphetamine synthesized by
the reductive amination of benzyl methyl ketone |
| Name |
Remarks |
Ref |
| Benzyl methyl ketone |
a |
24 |
| N-Acetylamphetamine |
c |
41 |
| Dibenzylketone |
e |
24 |
| N-(beta-Phenylisopropyl)-benzaldimine |
b |
40 |
| N-(beta-Phenylisopropyl)-benzyl methyl ketimine |
b |
40 |
| N,N-Di-(beta-Phenylisopropyl)amine |
b |
24 |
| 1-Oxo-1-Phenyl-2-(beta-Phenylisopropylimino)propane |
c |
41 |
| 2,4-dihydroxy-1,5-diphenyl-4-methylpentene |
c |
16 |
| Table 4. Impurities found in methamphetamine synthesized
by the reductive amination of benzyl methyl ketone |
| Name |
Remarks |
Ref |
| Benzyl methyl ketone |
a |
24 |
| Amphetamine |
c |
46 |
| 1-Phenyl-2-propanol |
b |
46 |
| N,N-Dimethylamphetamine |
c |
46 |
| Dibenzylketone |
e |
24 |
| Table 5. Impurities found in amphetamine synthesized with
the Leuckart reaction |
| Name |
Remarks |
Ref |
| Methylamine |
a |
24 |
| Formic Acid |
a |
24 |
| N-Methylformamide |
a |
24 |
| Benzyl Methyl Ketone |
a |
24 |
| Amphetamine |
c |
24 |
| N,N-Dimethylamphetamine |
c |
24 |
| N-Formylamphetamine |
b |
24 |
| N-Formylmethamphetamine |
d |
24 |
| Dibenzylketone |
e |
24 |
| alpha-Benzyl-N-methylphenethylamine |
c |
24 |
| N,N-Di-(beta-phenylisopropyl)amine |
c |
24 |
| N,N-Di-(beta-phenylisopropyl)methylamine |
c |
24 |
| Table 6. Impurities found in amphetamine synthesized with
Ephedrine |
| Name |
Remarks |
Ref |
| Benzyl Methyl Ketone |
a |
24 |
| 1,2-Dimethyl-3-phenylaziridine |
c |
20 |
| 1-Phenyl-2-methylaminopropanone |
b |
20 |
| Ephedrine |
a |
20 |
| 1-Chloro-1-phenyl-2-methylaminopropane |
b |
20 |
- Starting Material
- Intermediate Product
- Product of Side Reaction
- "Route Specific" Impurity
- Impurity in Starting Material
- Found in Phenylnitropropene route only
Conclusions
On the basis of the number and combination of impurities present in the drug preparation and in the case of Leuckart amphetamine c.q. methamphetamine of
"reaction specific" impurities, we can trace the synthesis of amphetamine or methamphetamine followed by the illegal producers. Furthermore it is possible
to decide (based on the similarity of impurity patterns) that illicit amphetamine preparations originate from the same production batch [34]. In the cited
study on the Leuckart reaction of amphetamine, the effects of the reaction conditions on the production of known impurities were investigated and the
possibilities examined for different chemists to produce amphetamine with the same impurity profile by rigidly following the same detailed synthetic
directions. Strong support was found in this study for the above stated assumption. Nonetheless the assumption of the equality of batches is tied to the
presence of quite a lot of impurities, in which the Leuckart reaction excels, because of its condensation character, where numerous reaction pathways can
be followed. With other types of syntheses, impurity signatures can give less information content, as fewer impurities can be present, due to the
possibilities for a given reaction. The meaning of equality of profiles under such circumstances should, of course, be approached by another standard of
value than in Leuckart type synthesis of amphetamine.
The fact that the identity of the impurities are known will stimulate studies in other unexplored areas, for instance, the acute toxicity of
alpha-benzyl-N-methylphenethylamine and alpha-benzylphenethylamine [30]. The abenzyl components appeared to have a greater CNS stimulation at the brain
stem and cord levels than amphetamine. Further studies in this field are expected to appear in due time.
Recent interest has been focused on the nature and level of inorganic trace impurities present in the final product of the methamphetamine synthesis.
By using inductively coupled plasma mass spectrometry (ICP-MS) or a combination of ion chromatography and ICP-MS [19,39] several inorganic trace impurities
in methamphet- amine were detected. It appeared even possible to differentiate in this way between two methods of synthe- sizing methamphetamine. It is
expected that further developments in this aspect will take place in due time.
References
- Alexander ER, Misegades ML: A low pressure reductive alkylation method for the conversion of ketones to primary amines; J Am Chem Soc 70: 1315; 1948.
- Alles GA: dl-Beta-Phenylisopropylamines; J Am Chem Soc 54: 271; 1932.
- Clandestine manufacture of substances under international control, United Nations, Vienna; 1987; ST/NAR/10.
- Commercial Solvents Corporation: Catalytic Reduction of Nitro Olefins; US Patent 2.647.930; 1953.
- Couturier PL: Action des ddrivdsorganomagndsiens mixtes; Ann Chim 10: 559; 1938.
- Crossley FS, Moore ML: Studies on the Leuckart reaction; J Org Chem 9:5291; 1944.
- Eight Peak Peak Index of Mass Spectra; The Mass Spectrometry Data Centre, The Royal Society of Chemistry: Nottingham; 1983.
- Frank RS: The clandestine drug laboratory situation in the United States; J Forensic Sci 28:18; 1983.
- Gairaud CB, Lapin, GR: The synthesis of omega-nitro styrenes; J Org Chem 18:1; 1953.
- Great Lakes Carbon Corporation: Reduction of Arylnitro-alkenes; United States Patent 3.458.576; 1969.
- Groot-Wassink BH, Duyndarn A, Jansen ACA: Synthesis of amphetamine; J Chem Ed 51:671; 1974.
- Haley TJ: Desoxyephedrine - a review of literature; J Pharm Ass Sci Ed 36:161; 1947.
- Haskelberg L: Aminative reduction of ketones; J Am Chem Soc 70:2811; 1948.
- Hey DH: dl-ß-Phenylisopropylamine and related compounds; J Chem Soc 18; 1930.
- Huizer H, Brusee H, Poortman-van der Meer AJ: Di(ß-phenylisopropyl) amine in illicit amphetamine; J Forensic Sci 30:427; 1985.
- Huizer H, Theeuwen ABE, Verweij AMA, Sinnema A, vd Toorn JM: Impurities in illicit amphetamine; J Forensic Sci Soc 21:225; 1981.
- Humphreys IJ: The work of the Drugs Intelligence Laboratory, Home Office Forensic Science Service; Bull Narcotics 36:33; 1984.
- Jaeger FM, van Dijk JA: Preparation of 2-phenylisopropylamine (benzedrine) the isomeric 1-phenylpropylamine and 3-phenyl-1,2-propanediamine and the resolution of these bases into their optical antipods; in Proceeding of the Section of Sciences of the Koninklijke academie van Wetenschappen; Amsterdam; 44:26; 1941.
- Kishi T: Analysis of trace elements in methylamphetamine hydrochloride by inductively coupled plasma-mass spectrometry; J Res Natl Bur Stand (U.S.) 93:469; 1988.
- Kishi T, Inoue T, Suzuki S, Yasuda T, Oikawa T, Niwaguchi T: Analysis of impurities in methamphetamine; Eisei Kagaku 29: 400; 1983.
- Kotera K, Okada T, Miyazaki S: Aziridine formation by reduction of ketoximes with lithiumaluminiumhydride. Dibenzylketoxime and its O-substituted derivations; Tetrahedron 24:6177; 1968.
- Kram TC: Identification of an impurity in illicit amphetamine tablets; J Pharm Sci 66:443;1977.
- Kram TC: Reidentification of a major impurity in illicit amphetamine; J Forensic Sci 24:596; 1979.
- Kram TC, Kruegel AV: The identification of impurities in illicit methamphetamine by GC/MS and NMR; J Forensic Sci 22: 40; 1977.
- Lambrechts M, Rasmussen KE: Use of bonded-phase silica sorbents for rapid sampling of impurities in illicit amphetamine for high-performance liquid chromatographic analyses; J Chromatogr 331:339; 1985.
- Lambrecht M, Tönnesen F, Rasmussen KE: Profiling of impurities in illicit amphetamine samples by high performance liquid chromatography using column switching; J Chromatogr 369:365; 1986.
- Larsen E: Notiz über die Reduktion von Oximen mit Lithiumaluminiumhydrid; Svensk Kem Tid 61:242; 1949.
- Lomonte JN, Lowry WT, Stone IC: Contaminants in illicit amphetamine preparations; J Forensic Sci 21:575; 1976.
- Moore ML: The Leuckart reaction; in Adams R (ed): Organic Reaction, Vol V; John Wiley: New York; p. 301; 1949.
- Noggle Jr TF, Clark RC, Davenport TW, Coker ST: Synthesis, identification, and acute toxity of alpha-benzylphenetylamine and alpha-benzyl-N-methylphenethylamine, Contaminants in clandestine preparation of amphetamine and methamphetamine; J Assoc Off Anal Chem 68:1213;1985.
- Purdue Research Foundation: Process for the Reduction of Arylnitroalkenes; US Pat 2.233.823 (1939)
- Recommended Methods for Testing Amphetamine and Methamphetamine; United Nations: Vienna; 1987; ST/ NAR/9.
- Sanger DG, Humphreys IJ, Ardrey RE: Internal Report 258; Home Office Central Research Establishment: Aldermaston; 1978.
- Sanger DG, Humphreys, IJ, Patel AC, Japp M, Osborne, RGL: The significance of gas chromatographic impurity patterns obtained from illicitly produced amphetamine; Forensic Sci Int 28:7; 1985.
- Sinnema A, Verweij AMA: Impurities in illicit amphetamine, a review; Bull Narcotics 33: 37; 1981.
- Schwoegler EJ, Adkins H: Preparation of certain amines; J Am Chem Soc 61: 3499; 1939.
- Stenhagen E, Abrahamson A, McLafferty FW: Atlas of Mass Spectral Data Vol I; Interscience: NY 1969.
- Strömberg L, Maehly AC: Advances of Chemical Signature Analyses of Drugs; in Proceedings of the International Symposium on Instrumental Applications in Forensic Drug Chemistry; United States Department of Justice: Washington, DC; p. 202; 1978.
- Suzuki S, Tsuchihashi H, Nakajima K, Matsushita A, Nagao T: Analysis of impurities in methamphetamine by inductively coupled plasma-mass spectrometry and ion chromatography; J Chromatogr 437:322; 1988.
- Theeuwen ABE, Verweij AMA: Impurities in illicit amphetamine 7. Identification of benzyl methyl ketone phenylisopropylimine and benzyl methyl ketone benzylimine in amphetamine; Forensic Sci Int 15:237; 1980.
- Theeuwen ABE, Verweij AMA: Verunreinigungen in illegalem Amphetamin 9. Identifizierung von N-Acetylamphetamin und 1-Oxo-1-phenyl-2-(phenyl isopropyl imino)propan; Archiv Krim 168:23; 1981.
- v.d. Ark AM, Sinnema A, Theeuwen ABE, v.d. Toorn JM, Verweij AMA: Impurities in illicit amphetamine 3. Isolation and identification of 2,4-dimethyl-3,5-diphenyl pyridine, 2,6-dimethyl-3,5-diphenyl pyridine and 4-methyl-5phenyl-2-(phenyl-meythyl)pyridine; Pharm Weekbl 113:41; 1978.
- v.d. Ark AM, Sinnema A, v.d. Toorn JM, Verweij AMA: Impurities in illicit amphetamine 2. Isolation and identification of 2-benzyl-2-methyl-5-phenyl-2,3-dihydropyridone; Pharm Weekbl 112:980; 1977.
- v.d. Ark AM, Sinnema A, v.d. Toorn JM, Verweij AMA: Impurities in illicit amphetamine 4. Isolation and identiftcation of 2-methyl-3-phenyl-6-(phenylmethyl)pyridine and 2,4-dimethyl-3-phenyl-6-(phenylmethyl)pyridine; Pharm Weekbl 113:341; 1978.
- v.d. Ark AM, Theeuwen ABE, Verweij AMA: Impurities in illicit amphetamine 1. Isolation and identification of some pyrimidines; Pharm Weekbl 112:977; 1977.
- v.d. Ark AM, Theeuwen ABE, Verweij AMA: Verunreinigungen in illegalem Amphetamin, Identifizierung von Phenylpropanol-2, Amphetamin und N,N-Dimethylamphetamin in Methylamphetamin; Archiv Krim 162:171; 1978.
- v.d. Ark AM, Verweij AMA, Sinnema A: Weakly basic impurities in illicit amphetamine; J Forensic Sci 23: 693; 1978.
- Wallach O: Über Methylamin; Ber 24: 3992; 1891.
|