An Evaluation of the Potential for Clandestine
Manufacture of 3,4-Methylenedioxyamphetamine (MDA)
Analogs and Homologs
by T. A. Dal Cason
Journal of Forensic Sciences, Vol. 35(3), 675-697 (1990)
HTML and graphics by Rhodium
To circumvent statutes enacted to control the use of various dangerous drugs (controlled substances), clandestine laboratory operators will sometimes make minor alterations in the molecular structure of a parent compound. These structural changes are reflected in the chemical nomenclature of the new analog or homolog, and, in the past, had effectively removed it from the purview of the law. Such modifications, at least in the case of 3,4methylenedioxyamphetamine (MDA), do not appear to be haphazard. They are made by design and are frequently based on information published in legitimate chemical and medical journals. A number of MDA analogs and homologs have been reported, and a much larger number are theoretically possible. To aid the identification of new MDA derivatives, it would be useful if the forensic chemist had a predictive scheme. This scheme could be used to target the most likely candidates at the commencement of the analysis or to supplement analytical data as it was acquired. Intuitively, there are three questions which should govern the appearance of new substituted MDA compounds: (1) Will the synthesized MDA derivative have central nervous system (CNS) activity? (2) is there a suitable method of synthesis? (3) Are the required precursors available? Obviously, compounds without some physiological effect will not intentionally be produced (however, see ref 41, pp 71 and 74, and ref 61, p 393 for unintentionally produced analogs). Syntheses which must be newly designed, or for which the published prototypes require a large number of steps, necessitate a high degree of skill, use specialized equipment, or result in low yields, will be poor candidates for clandestine manufacture of MDA analogs. Finally, if the precursor chemicals required are not commercially available (or are economically impractical) and must themselves be synthesized by the clandestine laboratory operator, then the manufacture of the corresponding analog is not likely. Each of these areas is important and will be dealt with separately. CNS ActivityOf the three areas to be considered, CNS activity is certainly the most difficult to evaluate. The estimations of potential CNS activity presented here are based on a variety of substituted phenethylamines and are not limited to MDA and its immediate derivatives. In addition, the CNS studies cited employed both human subjects and a variety of laboratory animals for determination of activity. Although human studies provide obvious advantages, man [1,2] and animals |1-4] each present problems in testing and evaluation of drug-induced activity. For example, the choice of animal species can affect the observed response to a given drug [2,5] and lead to results which appear contradictory. Similarly, extrapolation from animal studies to man may not produce an absolute correlation in either degree or type of CNS activity.Terminology [1], consensus as to what constitutes a particular category of action [1,3], and dosage-dependent responses [1-5] may each pose problems in the evaluation process. For the forensic chemist primarily concerned with compound identification, the distinction between terms used to define the drug-induced activity may be of small consequence. However, without some demonstrable and desirable pharmacological effect, new analogs will be of no value to those who illegally use controlled substances. To determine which drugs may elicit a particular CNS response, the clandestine chemist has at his or her disposal the same primary source of information as the forensic chemist: animal or clinical reports from legitimate scientific and medical journals. A review of this literature will permit reasonable evaluations of which compounds may be likely candidates for clandestine synthesis. The CNS activity initiated by substituted phenylisopropylamines covers a spectrum of physiological actions from the purely stimulant activity found in amphetamine to the purely hallucinogenic activity of 2,5-dimethoxy-4-methylamphetamine (DOM) [1,6-11]. Small changes in the structure of a molecule, such as addition or change of location of a substituent, can markedly alter, or abolish, the CNS action of the parent compound. The correlation between a particular aspect of molecular structure and the physiological activity of the compound has been widely studied in an effort to synthesize improved pharmaceuticals [10,11]. These structure-activity relationship (SAR) studies attempt to mimic some desirable physiological response to one compound by manipulating the molecular fragment believed responsible for that action onto, or within, a parent molecule. Experimentally, these molecular modifications must be evaluated one at a time since the observed effects may differ in qualitative or quantitative aspects from the anticipated results and are not necessarily additive. Although SARs have proven quite useful, it should be kept in mind that they are not infallible. Several examples follow which illustrate some of the anomalies which have been encountered using SARs. The compound 3,4-MDA has been established as having both stimulant and hallucinogenic properties [7-9,12,13] (MDA possesses a chiral center, and can exist as (R), (S), and (R,S) configuration. The S (+) enantiomer appears responsible for the stimulant effects of racemic (R,S)MDA, whereas hallucinogenic activity is attributed to the R (-) enantiomer [7,13]). In "drug discrimination" [3,4] studies using rats, relocation of the methylenedioxy bridge to give 2,3-MDA, produced an isomer which is reportedly only one-fifth as active as 3,4-MDA [9]. This isomer elicited a response in the animals indicating that they recognized the compound as 3,4-MDA. Remarkably. however, rats trained to identify either stimulant or hallucinogenic effects could distinguish neither in 2,3-MDA [8,9]. N-Methylation of MDA, to give N-methyl MDA, de creases the duration of action of the analog [14] and appears to minimize or abolish the hallucinogenic aspect [6,12-15]. N-Ethylation of MDA produces an analog which exhibits neither stimulant nor hallucinogenic effects in contrast. N-ethylation of amphetamine slightly reduces potency while retaining the stimulant effect of the parent compound. Homologation of N-methyl MDA, to a butyl side chain, instills new CNS properties termed "entactogen" [14,16] and provides a compound devoid of hallucinogenic activity with little or no stimulant effect remaining [14]. 3,4-Methylenedioxyphenethylamine (MDPEA), the alpha-demethylation derivative of MDA. is without central effect in man [1,13] at 200 mg. These examples provide a prelude to the following discussion and illustrate the difficulty in predicting the effect that small structural changes will have on human CNS activity. With this in mind, the following SAR review should serve as a guide for assessing the potential, or lack of potential, for some form of CNS activity. Ideally, the assessments made by the forensic chemist, even if incorrect, will parallel those of the clandestine chemist. Table 1 presents many of the possible substitution patterns for MDA. Throughout this discussion 3,4-MDA will be considered the parent, or reference, compound (R1 = CH3, R4 = CH2, R2, R3, R5 to R11 = H), upon which the indicated modification is effected. When nonbridged phenylisopropylamines such as ephedrine, DOM, phentermine, or cathinone are being described, it should be recognized that the 3 and 4 ring positions in Table 1 are occupied by hydrogen or, as appropriate, the substituents shown for R7, R8, and R9. TABLE 1 - Substitution patterns for clandestinely produced MDA analogs or homologs or both 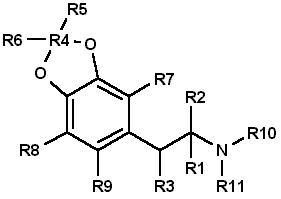 R1, R2 = H, CH3, C2H5 and R1 and R2 may be the same or different R3 = H, OH, CH3, Cl, Br, O= R4 = C, C2H2, C3H4, if R5 = R6 = H R5, R6 = H, CH3, C2H5, C3H7 when R4 = CR5R6 and R5 = H or R5 = R6 R7, R8, R9 = H, CH3, C2H5, C3H7, OCH3, OC2H5, Br, SCH3, and when R7, R8 and R9 are the same or different R10 = H, OH R11 = H, CH3, C2H5, i-C3H7, n-C3H7 or R10 = R11 = CH3 Length (R1) and Branching (R2) of Side ChainThe three-carbon side chain (R1 = CH3) provides the most active compounds [17-21]. Decreasing the side chain by one carbon (R1 = H) produces phenethyl derivatives which exhibit reduced stimulant or hallucinogenic activity or both [1,2,8,13]. increasing the side chain of the hallucinogenic amphetamines by one carbon (R1 = C2H5) to give butane analogs causes abolition of hallucinogenic activity [14,22]. However, the N-methylated butane derivative of MDA is reported to have novel CNS effects [14,16] with neither stimulant nor hallucinogenic properties. In amphetamine, (R1 = CH3; R. = H) branching produces phentermine (R1 = R2 = CH3) and a resultant decrease in stimulant activity [10,11]. With the ethyl homolog (R1 = CH3; R2 = C2H5), CNS stimulation [11] is absent. An indirect assessment [23] of the corresponding methylenedioxy phentermine analog (R1 = R2 = CH3) indicates that the above trend of decreased stimulant activity with increased branching may also hold for this compound.Beta Substitution (R3)By analogy with the amphetamine/phenylpropanolamine [10,11,24] and methamphetamine/ephedrine pairs, hydroxy substitution (R3 = OH) of MDA could lead to a decrease in CNS stimulant activity. Oxidation of the hydroxy group of phenylpropanolamine or ephedrine produces the aminoketones (R3 = 0= ) cathinone and methcathinone, respectively. Examination of cathinone in rats [4,8] and methcathinone in mice [6,11] indicate a stimulant effect similar to that of their nonoxygenated counterparts. Potentially, beta keto MDA and beta keto MDMA could retain stimulant activity similar to the unbridged parent compounds.Substitution of a methoxy group at the beta position [R3 = OCH3] on MDPEA has been reported to give an hallucinogenic homolog of equal potency with MDA [24]. in view of this, a clandestine laboratory chemist may assume that beta methoxy substitution on MDA would also yield an hallucinogenic analog and target this compound for synthesis. The beta chloro and bromo derivatives of amphetamine have been prepared as synthetic intermediates but apparently have not been evaluated for CNS activity. Masking or decreasing the polarity of the atom or group attached to the beta carbon can lead to increased CNS stimulation [24]. It may be anticipated that beta halo derivatives would have some CNS activity. Alteration of the Alkyldioxy Bridge (R4, R5, R6)The alkyldioxy bridge may occupy either of two isomeric positions on the phenyl ring: 3,4 or 2,3. When substitutents are added to the ring, the 3,4 position is equivalent to the position and the 2,3 position is identical to the 5,6 position. The appropriate choice of numbers will be determined by the location of substituents R7, R8, and R9. Both isomers and their various methoxy derivatives (R7, R8, and R9, see the following) [1,2,11,25] appear to stimulate hallucinogenic/psychotomimetic activity to some degree. Insertion of methylene groups into the methylenedioxy bridge (R4), to give an ethylenedioxy (-O-CH CH2-O-) or trimethylenedioxy (-O-CH CH CH -O-) compound, leads to a decrease in CNS effectiveness [17,18]. 3-Methoxy-4,5-ethylenedioxyamphetamine is reported to have one third the hallucinogenic activity of MDA [2]. Addition of one methyl group (R5 = CH3, R6 = H) to the methylenedioxy bridge diminishes activity, while two methyl groups (R5 = R6 = CH3) inactivates the parent molecule [26].Ring Substitution (R7, R8, R9)Addition of one methoxy group (R7 = OCH3, R8 = R9 = H) in either ortho ring position (2-methoxy or 6-methoxy) greatly increases the hallucinogenic activity of MDA [1,2,11,27,28]. Meta substitution (5-methoxy) produces a less potent isomer [1,2,11,27,28] having CNS effects which could not be accurately defined as either psychotomimetic or hallucinogenic [25]. Putting a methoxy group at the 2 position of the homolog MDPEA yields a "mood elevator" [1] of greater activity than 3,4-MDA; a methoxy group at the 5 position decreases the psychotomimetic activity induced by this substituent [1,2]. Dimethoxylation of 3,4-MDA can produce either 2,5-dimethoxy-3,4-MDA or 2,3-dimethoxy-4,5-MDA, each of which has greater hallucinogenic [1] activity than the parent compound [1,2,11]. Bromination of 3,4-MDA at the 6 position (that is, 2-Br-4,5 MDA) results in a less active hallucinogen with an amphetamine-like stimulant activity [29].Table 1 presents several substituents that are possible candidates for ring addition [19,29-36]. Exploration of these substituents has primarily been undertaken using 2,5-dimethoxyamphetamine (2,5-DMA) as the parent compound with substitution frequently occurring at the 4 (para) position. This position yields particularly active hallucinogenic analogs [33-36]. Substitutents that are more resistant to metabolic oxidation will generally produce greater potency [34,36]. The para position is already occupied by part of the methylenedioxy bridge in 3,4-MDA prohibiting substitution and making a comparison by analogy impossible. The compound 4-methoxy-2,3-MDA [1,2] is the single 4-substituted methylenedioxy compound which has been evaluated. It exhibits activity and potency approximately equal to 3,4-MDA [1,2] and is five times as potent as 2,3-MDA [9]. Attachment of other groups listed in Table 1 may also produce some compounds with CNS activity. In general, substitution with ethoxy [30] or thiomethyl [31,32] groups at ortho or meta positions in substituted phenylisopropylamines resulted in less active compounds. In para this is, 4)-substituted 2,5-dimethoxyamphetamines, moving the methyl, methoxy, or bromo substituent to the meta (that is, 3) position yielded less active analogs 18]. N-Substitution (R10, R11)Introduction of a methyl (R10 = CH3, R11 = H) or an ethyl (R10 = CH3, R11 = H) group to the nitrogen atom provides CNS active compounds [20,21,37]. in general, N-methylation of phenylisopropylamines, which are hallucinogenic (for example, DOM), decreases their potency; however, it has little effect on compounds which have stimulant activity (for example, amphetamine) [6,8,12]. The N-propyl and N-isopropyl analogs have not shown CNS activity in humans at dosages of 160 mg [21]. The tertiary amine analog, N,N-dimethyl MDA, has also proved ineffective at the 160-mg level [21] but may produce CNS effects at 0.5- to 1-g doses [38]. The N-hydroxy analog (R10 = OH, R11 = H) possesses CNS activity comparable to MDA [20]. The N-alkyl-N-hydroxy MDA derivatives apparently have not been evaluated or synthesized. Two phenethylamine derivatives, N-methyl- and N,N-dimethyl-3,4-MDPEA, give no indication of CNS response at low dosages [11].Examination of these factors raises an expectation that future MDA analogs will have a 3 carbon straight chain and that the oxygen atoms at the 3,4 (that is, 4,5) positions of the phenyl ring will be bridged by an unsubstituted methylene group. Monomethoxy substitution at the 2 and 6 position and dimethoxy substitution at the 2,5 or 5,6 positions of 3,4-MDA will be obvious choices for experimentation by clandestine chemists. Replacement of an amino hydrogen with small alkyl groups or addition of oxygen to give N-hydroxy-MDA will yield analogs with proven CNS activity. A number of these N-substituted analogs have been reported by forensic science drug laboratories. Placing a methoxy group or keto oxygen on the beta carbon atom has the potential to provide compounds approximately equal to MDA in quantitative and qualitative effect. Less effective CNS active products may result with the above substituent combinations on 3,4-MDPEA, but other variables being equal, these lower-potency drugs would not be the products of first choice for the clandestine chemist. SynthesisThe second important area is selection of the synthetic method (Figs. 1-4 and Table 2). in general, the single-step techniques, which require little knowledge of chemistry (Schemes 1 through 4, Fig. 1), are the ones most likely to be used in clandestine laboratories. An experienced, innovative chemist may be able to devise novel syntheses or employ more difficult synthetic route. However, the ready availability of established techniques should ensure their continued dominance in clandestine laboratory applications. In particular, the wide variety of reactions successfully used to synthesize amphetamine and its analogs, homologs, and derivatives provides a fertile area for investigation by the clandestine chemist.TABLE 2 - Products (I-VI) and nitrogen sources (a-m) for figures 1-4 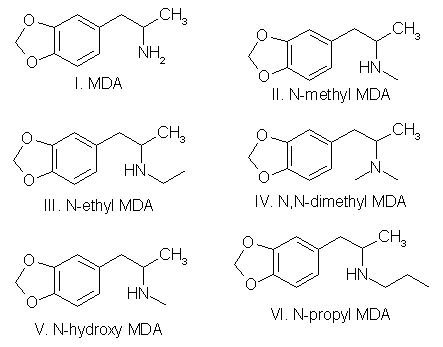
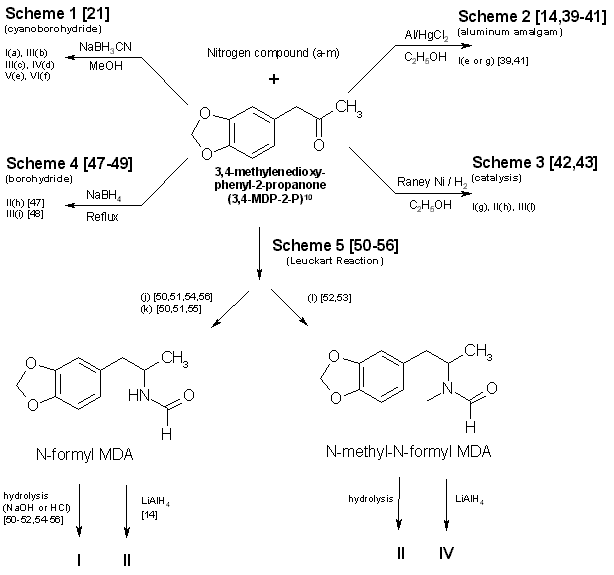
Figure 1 - Reactions Using 3,4-MDP-2-P as a precursor in synthesis of MDA and some analogs A number of the references provided herein describe the synthesis of various phenylisopropylamines other than MDA. These additional references will enable forensic scb ence chemists to examine the types of reactions, and the range of parameters, available for use and modification by the clandestine chemist. Appropriate substitutions of reactants in many of these syntheses can permit a variety of MDA analogs and homologs to also be prepared. However, the choice of substitutions in a particular reaction is not always unrestricted. For example, the aluminum amalgam reduction (Scheme 2, Fig. 1) [14,39-41] initially described the production of methamphetamine. Substituting ammonium hydroxide and 3,4-methylenedioxyphenyl-2-propanone (MDP-2-P) [41] for aqueous methylamine and phenyl-2-propanone (P-2-P), will yield the expected product, MDA. If hydroxylamine hydrochloride is used as the amine source in an attempt to make N-hydroxy MDA, only unsubstituted MDA is recovered. Braun, et al. (Scheme 1, Fig. 1) [21] successfully used N,N-dimethylamine hydrochloride (HCl) to synthesize N,N-dimethyl MDA. Yet simple substitution of N,N-diethylamine HCl or N-methyl-N-ethyl-amine HCl in the same reaction fails to provide the diethyl [21] or the methyl ethyl analogs. Attempts (Raney Ni (W-2), 95% EtOH, MDP2P, sec amine and H2 (55 psi) reacted in a Parr hydrogenating apparatus at room temp) at this laboratory to use catalytic means [42,43] to produce the above analogs through use of the secondary amines as free bases (Scheme 3 Fig. 1) were also unsuccessful. Several modifications of Schemes 1-8 (Figs. 1-3) have already been encountered in clandestine laboratories synthesizing a variety of phenylisopropylamines. An example of a potential synthesis modification [44] to produce MDA in the clandestine laboratory can be formulated from the Ritter reaction (Scheme 7, Fig. 2), which originally detailed the manufacture of amphetamine from allylbenzene [45]. Substitution of 3,4-methylenedioxyphenyl-2-propanol and hydrogen cyanide [44] for the listed reactants in Scheme 7 [46] could result in successful synthesis of MDA. In this instance, the concomitant risks from using hydrogen cyanide must be fully recognized. Of the synthesis techniques presented, Scheme 1 (Fig. 1) [21] is probably the most attractive. It requires no knowledge of chemistry, has a wide applicability, offers little chance of failure, produces good yields, does not require expensive chemical apparatus or glassware, and uses currently available (and easily synthesized) precursors. Scheme 2 (Fig. 1) [14,39-41] has most of the desirable features of Scheme 1, but may not be as versatile in terms of the amines which can be employed. Scheme 3 (Fig. 1) [42,43], catalytic hydrogenation (Raney Nickel), is frequently successful at atmospheric pressure but is most effective under increased pressure. This requires purchase or construction of a suitable hydrogenation unit. Use of pyrophoric activated Raney Nickel (or other active catalysts) and hydrogen are drawbacks with this method. Nonetheless. a significant number of clandestine laboratories have successfully used catalytic hydrogenation for amphetamine and methamphetamine manufacture. Reduction of the intermediate imine (ketone + amine => imine) using sodium borohydride, Scheme 4 (Fig. 1) [47-49], is a relatively simple reaction. Harsher conditions (that is, reflux) [48] may be required when using sodium borohydride as the reducing agent than in the techniques using aluminum amalgam or sodium cyanoborohydride. A substantially lower yield is also obtained. Scheme 5 (Fig. 1) [50-56]. the Leuckart reaction, is a more difficult synthesis than the other ketone-based schemes which use a single reaction to obtain the desired product. Refluxing may be necessary to form the N-substituted intermediate and, subsequently, hydrolysis or reduction produces the final product. Longer reaction times are inherent in the process, yields are expected to be less than the first three methods, and chemical apparatus must be used. 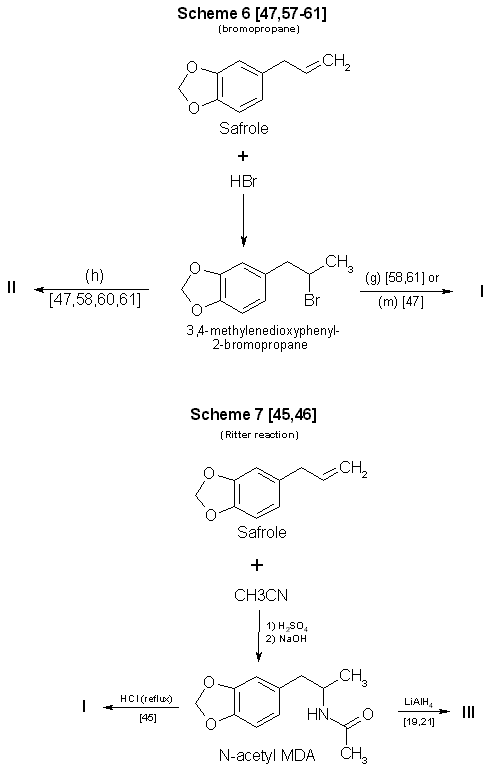
Figure 2 - Reactions Using safrole as a precursor in synthesis of MDA and some analogs Scheme 6 (Fig. 2) also uses a two-reaction sequence to produce the final product. Although safrole is an inexpensive and readily available starting material, preparation of the intermediate 1-(3.4-methylenedioxyphenyl)-2-bromopropane [47,57-61] is a process that may be time-consuming [47,57,60] and potentially hazardous [47]. In addition, the yield is no better than with Scheme 4. Reported yields for amphetamine using the Ritter reaction [45] (Scheme 7, Fig. 2) were less than 30%. As in Schemes 5 and 6, a two-step synthesis is involved. Safrole is first converted to the immediate precursor N-acetyl MDA. This compound must then be either hydrolyzed or reduced to yield the desired product. The process is time-consuming, requires a degree of laboratory skill, and may give low yields. 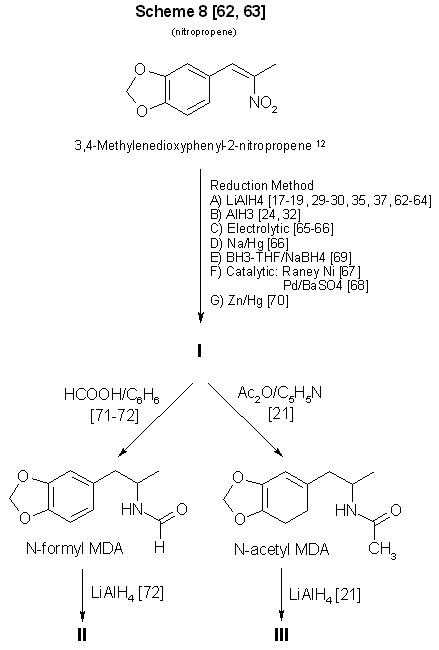
Figure 3 - Conversion of 3,4-methylenedioxyphenyl-2-nitropropene to MDA and some analogs 12 See ref 86-87 for methods of preparing MDP2P oxime from this compound. A substantial amount of literature has been devoted to the synthesis and reduction of nitroalkenes [17-19,24,29,30,32,35, 37,62-70] (Scheme 8, Fig. 3, and Scheme 10, Fig. 5). The compound 1-(3,4-methylenedioxyphenyl)-2-nitropropene (beta-nitroisosafrole) commercially available and easily synthesized [62,63]. However. preparation of N-substituted analogs requires that the reduction product, MDA, be further processed [21,71,72]. it is unlikely that the clandestine chemist would continue to manipulate chemically a controlled substance to produce an analog of similar monetary value and marketability. If the appropriate piperonals are available to react with nitroethane, the Knoevenagel-Walter condensation, will permit the synthesis of ring-substituted analogs [24,29] through nitropropene intermediates (Fig. 5). 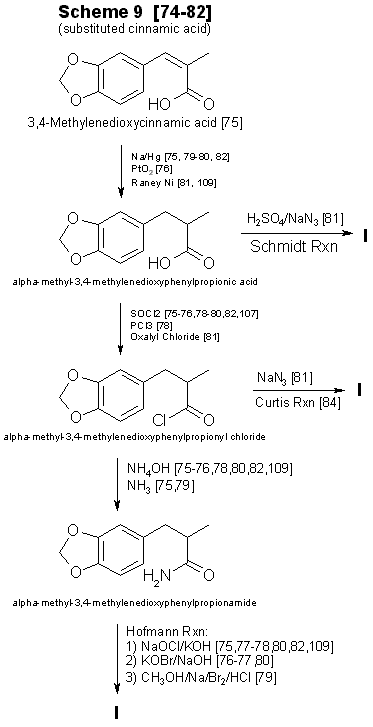
Figure 4 - Techniques for converting substituted cinnamic acids to to MDA Currently, alpha-methyl-3,4-methylenedioxycinnamic acid (Scheme 9, Fig. 4) and its ring substituted derivatives are not commercially available [73]. Synthesis [74-82] of the substituted cinnamic acid precursors requires several steps having various modifications [79,80,83,84], in addition to the reaction sequence necessary for preparation of MDA or its analogs [75,78,82]. This technique requires substantial laboratory skills, and the concomitant low yields imply that his method will infrequently be encountered. One approach not delineated in Fig. I is the preparation of 3,4-methylenedioxyphenyl2-propanone oxime from the ketone [21,41,85]. Nitropropenes [86,87] have also been used to produce oximes. Even though MDP-2-P oxime [21,85] has been found in clandestine laboratories, the additional steps required for the synthesis and isolation of oximes prior to reduction [21,85-89] will limit their use for preparation of MDA analogs. Oximes have served as precursors for the synthesis of N-alkyl, N-hydroxy amines [90]. Based on synthesis considerations, Schemes 1-4 are the most likely to be used in clandestine laboratories. Schemes 5-9 are more time-consuming. require greater laboratory skills, generally result in poorer yields, and thus are less likely to be used. PrecursorsThe remaining topic is availability of precursors. As noted in prior sections of this paper, the literature citations provided here and in Table 5 are listed to illustrate general reaction types or variations in reactants and catalysts and do not necessarily delineate a synthesis which is specific for a methylenedioxy compound. This will provide the forensic science chemist with the widest possible acquaintance with potential clandestine laboratory synthesis modifications. Table 3 includes alternative names for some precursors and related compounds. The relationship between the five most often used precursors, safrole, isosafrole, piperonylacetone (that is, 3,4-MDP-2-P), piperonal, and beta-nitroisosafrole, is illustrated in Fig. 5 [17,37,54-56,62-65,79,86.87.91-105,107,108]. All five compounds are commercially available [73]. The first two are usually encountered as the starting materials in preparation of piperonylacetone, whereas piperonal generally serves as the primary precursor for beta-nitroisosafrole. Safrole, isosafrole, and piperonal may themselves be prepared in good to excellent yields from 1,2-methylenedioxybenzene through the 4-bromo intermediate [106]. Substitution of 1,4-benzodioxane as the starting material in that reaction should produce ethylenedioxy precursors.Several analog precursors suitable for substitution in Schemes 1-8 are available on the open market [73], although the selection is rather limited: 2,3-methylenedioxybenzaldehyde, 5-methoxysafrole, 5-methoxypiperonal, 6-nitropiperonal, and 2,5-dimethoxysafrole. The current price of these chemicals ranges between 1 dollar (6-nitropiperonal) to over 200 dollars per gram (2,3-methylenedioxybenzaldehyde). An abundance of literature exists on the preparation of the isomeric methoxypiperonals [107-111], 5-methoxysafrole [112], ortho-safrole [113], 5,6-dimethoxysafrole [114], alkylenedioxy bridges [26,106,109,110,115,116], and brominated phenethylamine-based compounds [29,106,117]. Ring-substituted alpha methyl-3,4-methylenedioxyhydrocinnamic acids 174,75,80] may be prepared from appropriate aldehydes (three steps) and converted into methylenedioxyphenylisopropylamine analogs through the multi-step synthesis shown in Scheme 9 (Fig. 4). In two instances cinnamic precursors are commercially available which would enable the clandestine-laboratory operator to avoid their lengthy synthesis. 3,4-Methylenedioxycinnamic acid [82] used in Step I of Scheme 9 yields 3,4-MDPEA as the final product. Oxidation of the second compound, alpha-methyl-3,4-methylenedioxyhydrocinnamic aldehyde, gives the appropriate precursor (Step 2, Scheme 9) for synthesis of MDA. The 2,3 isomer of methylenedioxycinnamic acid has been synthesized from ortho-piperonal, but efficiency of the reaction was not reported [118]. Using the appropriate reactants and making slight changes in reaction parameters [119124] for the preparation of beta-nitroisosafrole (Fig. 3) may permit the production of 1(3,4-methylenedioxyphenyl)-2-nitro-1-propanol. Nitro alcohols may be reduced to the amino alcohol with zinc and sulfuric acid [124], zinc and acetic acid [125], sodium amalgam [124,125], or by catalytic hydrogenation [124-126]. The conversion of amino alcohols to amines is accomplished directly by reduction with hydriodic acid [120,125] or indirectly through preparation of the 1-chloro intermediate, which is then catalytically reduced [126,127]. Phentermine (alpha,alpha-dimethylphenethylamine) and N-methylphentermine have both been synthesized from nitro alcohols [125-126]. The former drug has also been prepared by a modified Ritter reaction [44]. Each of these methods presents a potential synthesis approach for the production of methylenedioxy analogs of phentermine. Amino alcohols have also been prepared by using propiophenones [127-129] as starting materials. Considering this, 3,4-methylenedioxyphenyl-1-propanone (3,4-MDP-1-P) may prove suitable as a precursor for 1-hydroxy-MDA. Chromic acid oxidation [6] of this alcohol may lead to the 1-keto analog of MDA. Many of these precursor preparations have serious drawbacks. Synthesis of the 2methoxy and 5-methoxy piperonals by the procedure of Campbell et al. [110 requires the use of a heated reaction bomb and multiple steps, resulting in poor yields. Reasonable yields of 6-methoxypiperonal [110] might be obtained with this procedure if a commercially available intermediate 6-nitropiperonal [73] is purchased rather than synthesized. Another method for the synthesis [107,109] of 5-methoxypiperonal (myristicinaldehyde) requires an expensive precursor, 5-methoxysafrole (myristicin), for preparation [112] of the starting material, 5-methoxyisosafrole (isomyristicin). The synthesis of 5-methoxysafrole has also been described [109], but the process is encumbered by multiple steps and a poor yield. The first described preparation of 2-methoxysafrole (croweacin) used 2-hydroxysafrole [130], a compound which is not currently marketed. Use of sesamol to prepare 6-methoxy-MDA has also been reported [271], but exact details and the yield were not presented. The preparation of alkylenedioxy bridges from dihydroxy compounds [109 115,118] has generally suffered from moderately difficult synthesis or low yields. or both. Using cesium fluoride, Clark et al. [131] produced high yields (>80%) of 3,4-methylenedioxy compounds from catechol, 3-methylcatechol, and 3,4-dihydroxybenzaldehyde. This procedure with 2,3-dihydroxybenzaldehyde yields less than 50% [132] ortho-piperonal, Piperonal and 5-methoxypiperonal have been prepared in good yields from the corresponding dihydroxybenzaldehydes by catalyzing the reaction with copper oxide [116]. Involved and rather difficult syntheses [74,76,79,80] will probably deter the preparation of substituted alpha-methyl methylenedioxyhydrocinnamic acid precursors [75,109] and thus limit their use. Unless a 1-substituted derivative is desired as the final product, 3,4-MDP-1-P is also an unlikely precursor. Preparing MDA (or an N-alkyl analog) from this propiophenone requires a multi-step synthesis which has no advantage over the more facile procedures using 3,4-MDP-2-P. 3,4-MDP-2-P, unlike its amphetamine precursor counterpart phenyl-2-propanone (P-2-P), is not currently a controlled substance. A frequently overlooked source of precursors with potential importance are the essential oils [28,46,92,133-135] Sassafras oil (80 to 90% safrole), Indian dill seed oil (up to 53% dill apiol, that is, 2,3-dimethoxysafrole) [136], nutmeg oil (0.5 to 13.5% myristicin, that is, 5-methoxysafrole; 0.1 to 3.2% safrole) [107,135,137], mace oil (10% myristicin, some safrole) [135], and parsley seed oil (9 to 77% myristicin; 0 to 80% parsley apiol, that is, 2,5-dimethoxysafrole) [138] each contain suitable precursors for preparation of MDA or its mono- or dimethoxy derivatives. All of these essential oils have additional components which are of no value in the synthesis of MDA analogs. It may be possible to use these mixtures as starting materials without initial processing and then purify the resulting products to isolate the MDA analogs. The primary drawback for clandestine laboratory operators in using essential oils as precursors (with the exception of sassafras oil) is the unknown, and sometimes substantial, variation in concentration of the desired constituent between lots or commercial sources or both. The forensic science chemist, identifying a mixture [92,133-135,137,138] of ring-substituted methoxy MDA analogs or finding methoxy amphetamine contaminants, might suspect essential oils as precursors. Evaluation of the potential for MDA analog synthesis based on precursor availability (either commercial or synthetic) points to the conclusion that 3,4-MDP-2-P will be the precursor of choice for MDA and its N-substituted analogs. This precursor is commercially available, lends itself to a variety of synthesis, is not currently controlled, and, if necessary, can be fairly easily synthesized. The methoxy-substituted safroles, found in the essential oils, will serve as precursors for preparation of ring substituted 3,4-methylenedioxyphenylpropanones. These ketones will permit a series of monomethexy and dimethoxy analogs and their N-substituted derivatives to be prepared. TABLE 3 - Alternate names for MDA precursors and related compounds
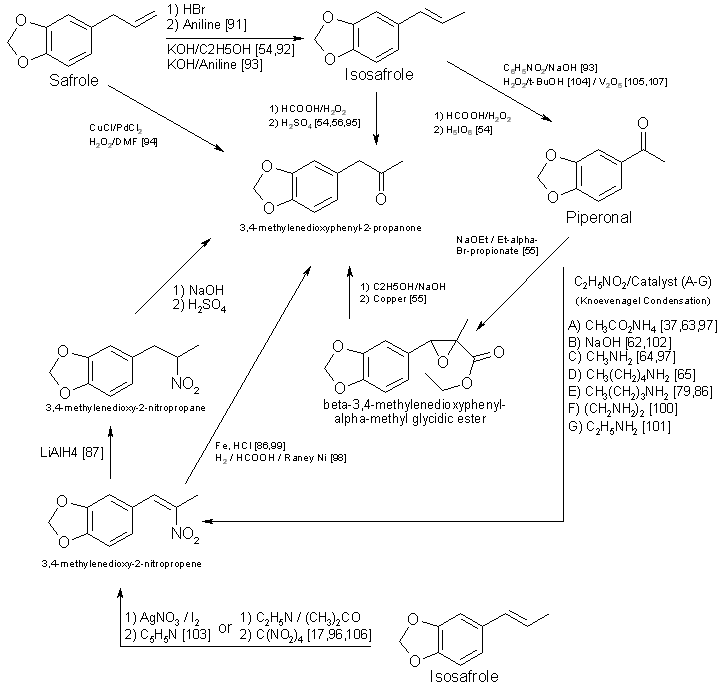
Figure 5 - Interrelationships of precursors used in the synthesis of MDA and its analogs ConclusionIntegrating the three components. the evaluations of potential CNS activity, synthesis method, and precursor availability, several likely, and unlikely, candidates for clandestine manufacture are suggested. Because of the lack of commercially available precursors and a reduced CNS activity, compounds containing the 2,3-methylenedioxy bridge, or expanded bridges in either position. are not likely candidates. Future analogs will almost certainly contain the 3,4-methylenedioxy bridge. Suitable precursors are commercially available for preparation of piperonylethylamines (that is, substituted piperonals with nitromethane) and piperonylbutylamines (that is, 3,4-methylenedioxyphenyl-2-butanone); however, the propyl side chain provides the most active analogs and would therefore be preferred. Nonetheless, substituted phenylethylamines have recently appeared in illicit drug traffic and the synthesis of substituted MDPEA analogs, although remote, is tenable.Preparation of substituted amino nitrogen analogs (Scheme 1) is relatively simple. Illicit drug exhibits containing the N-methyl, Methyl, N-n-propyl, N,N-dimethyl and N-hydroxy analogs have been reported. Bromination of the ring requires an additional synthesis step, and the resulting compounds are likely to be less active than the corresponding nonhalogenated compound. Brominated ring analogs are therefore unlikely to be manufactured. Using the essential oils as precursors, 3-methoxy-4,5-MDA (nutmeg oil, mace oil, or parsley seed oil); 2,5-dimethoxy-3,4-MDA (parsley seed oil); and 2,3-dimethoxy-4,5-MDA (dill seed oil) are ideal candidates for clandestine-laboratory synthesis. Since preparation of these analogs would most likely proceed through the synthesized ketones (Fig. 5), Scheme I would provide access to a series of their N-substituted homologs. Lack of commercially available precursors will probably prevent additional ring-substiuted analogs from being synthesized. Analogs which are synthesized and prove to be inactive will not be sustained by the underground market. The occurrence of such analogs will be transient, and, although of forensic science interest, will not become drug abuse problems. AcknowledgmentThe author would like to thank Mr. Frank Sapienza, Dr. Alexander Shulgin, and Dr. Richard Glennon for their invaluable comments and suggestions. The author is also indebted to Mr. Roger Ely for preparation of the figures and Ms. Tina Hellman for typing the manuscript.References
|