NaIO4/DMFA reagent for conversion of organic halides to carbonylsTetrahedron Letters 44, 1375-1377 (2003)ASCII by GC_MS, HTML by metanoid [ Back to the Chemistry Archive ] Abstract NaIO4-DMF oxidises various primary and secondary halides to the corresponding aldehydes and ketones under mild conditions (150°C/4060 min) in high yields (70-90%). Introduction Oxidation of organic halides to the corresponding carbonylcompounds is a well known transformation in organic synthesis. In 1949, the HassBender reaction was reported for the oxidation of halides.1 Several methods have been developed to carry out this conversion 2,3 such as the Sommelet reaction 4 which is limited to benzylic halides, the Krohnke reaction (pyridine followed by p-nitrosodimethylaniline) 55 and the Kornblum reaction (DMSO/NaHCO3) 6 which is limited to active halides and requires very high temperature. Various amine N-oxides 7-9 are also used for this oxidation. Masaki et al. 10 have reported the photooxidation of aryl bromides with mesoporous silica FSM-16. More recently, 2-dimethylamino-N,N-dimethyl aniline-N-oxide 11 was used for this conversion in high yield. All the above procedures are limited and required high temperatures. To circumvent all these obstacles, we planned to explore the use of NaIO4-DMF for the oxidation of halides to the corresponding carbonyl compounds. We wish to report here a new and convenient method for the preparation of aldehydes and ketones from the corresponding halides. The importance of sodium metaperiodate in organic synthesis prompted us to examine the behaviour of sodium metaperiodate and dimethyl formamide on different halogen compounds. Primary and secondary halides were reacted with NaIO4-DMF to give the corresponding aldehydes and ketones, respectively. It was found that different primary halides (Table 1) and secondary halides (Table 2) gave the corresponding carbonyl compounds in good yields in short time periods. Octyl bromide was converted into octanal in an 85% yield (Table 1, entry 1). Benzyl bromide (entry 2) and a substituted benzyl bromide (entry 3) gave the corresponding aldehydes in good yields. Different halomethyl naphthalenes (entries 4, 5 and 6) were also converted to the corresponding aldehydes in 8085% yields. Cinnamyl bromide and chloride (entries 7 and 8) also gave cinnamaldehyde in 90 and 84% yields in 50 and 55 min, respectively. Secondary halides gave the corresponding ketones, as shown in Table 2. For example, cyclohexyl bromide (Table 2, entry 1) gave cyclohexanone in an 84% yield. Bromodiphenyl methane was converted to benzophenone in 90% yield and chlorodiphenyl methane (Table 2, entry 3) took a little more time but gave benzophenone in 80% yield. 9-Bromofluorene (Table 2, entry 4) give 9-fluorenone in good yield in 45 min. The behaviour of this reagent towards the alfa-halocarbonyl compounds, i.e. phenacyl bromide was also examined, but to our surprise benzaldehyde was isolated instead of phenyl glyoxal. Phenacyl bromide might have undergone the same oxidation (Scheme 1) as primary halides to give phenyl glyoxal which is further oxidized to phenylglyoxalic acid which undergoes decarboxylation to give benzaldehyde (85% yield based upon GCMS). In conclusion, we have developed a new method to convert primary and secondary halides to the corresponding aldehydes and ketones, using for the first time the oxidising property of a mixture of NaIO4 and DMF. Typical reaction procedure Benzyl bromide (0.34 g, 2 mmol) was taken in a round bottom flask along with sodium metaperiodate (NaIO4) (0.42 g, 2 mmol). The above mixture was dissolved in 30 ml of N,N-dimethyl formamide (DMF). The reaction mixture was heated at reflux. The progress of the reaction was monitored by thin layer chromatography by comparison with the starting material (10% ethyl acetate in hexane). The reaction was completed in 40 min. The reaction mixture was cooled and treated with 20 ml of water and then extracted with ether (2×30 ml). The combined ether layers were dried over anhydrous magnesium sulphate (MgSO4), then filtered off and concentrated. GLC analysis of the reaction mixture showed the presence of benzaldehyde (80%). Purification by column chromatography on 60-100 mesh silica gel gave (0.18 g, 80%) of benzaldehyde. The formation of benzaldehyde was further confirmed by the melting point of its 2,4-dinitrophenylhydrazone derivative. 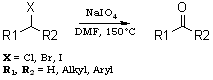
Scheme 1
Scheme 2
References [1] Hass, H. B.; Bender, M. L. J. Am. Chem. Soc. 1949, 7,1767-1769.
|