Diazomethane can be generated from a wide variety of precursors by adopting methods1-5 which employ dangerous and time-consuming distillations and collection of the gaseous reagent. A certain degree of safety may be attained by using special sets of distillation glassware which are commercially available at relatively high cost. Small quantities of diazomethane frequently employed in routine work are generally taken from a supply of the reagent, prepared as above, which has to be stored in the cold or is generated as required using either expensive commercial microapparatus or custom-made apparatus6.
The author has developed a technique which permits the rapid and safe generation of diazomethane and its simultaneous reaction with the substrate, using an apparatus which can be easily and cheaply assembled with ordinary glassware in a few minutes. This technique works equally on the mole, millimole and analytical scales.
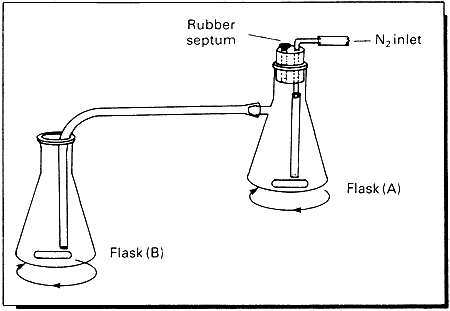
The apparatus consists of a Buckner-type conical flask (A) fitted with a magnetic bar and a rubber stopper. The stopper bears two holes: the first one is closed with a rubber septum and the second one accommodates a L-shaped glass tube which is connected at both ends to polythene tubing. The side arm of flask (A) is connected to an Erlenmeyer flask (B) through polythene tubing, as shown in the Figure. Flask (A) is charged with the required amount of p-toluenesulphonylmethylnitrosamide (Diazald7) suspended in 5 times its weight of ethanol (it is assumed that 1g of fresh Diazald generates ca 3 mmol of diazomethane). Flask (B) is charged with the substrate dissolved in a suitable solvent and cooled with an ice-bath. A good stream of nitrogen is then allowed to pass through the whole system, while a concentrated aqueous solution of sodium hydroxide is dripped into flask (A) at intervals via the rubber septum by means of a hypodermic syringe. After a few seconds yellow diazomethane begins to evolve and to pass into flask (B) where reaction with the substrate occurs. Sodium hydroxide solution is added till the yellow colour in flask (A) is discharged.
Experimental
The following example illustrates the method:
Flask (B) is charged with phenoxy-acetamidopenicillanic acid 1-oxide (20g, 55 mmol) dissolved in methylene dichloride (400 mL) and ethanol (10 mL) and cooled to 0°C. Flask (A) is charged with Diazald (18g, 84 mmol) (corresponding to ca 59 mmol diazomethane) suspended in ethanol (110 mL) and the mixtureis stirred while nitrogen is allowed to flow into the system. Sodium hydroxide solution is added dropwise until the Diazald dissolves, then at a rate of 1 mL every 30 sec. till the yellow colour in the flask (A) is discharged. TLC monitoring of the solution in flask (B) showed complete methylation of the acid.
This method has been widely adopted in this and other laboratories8 for several years and, due to its simplicity and safety, may be performed by unqualified technicians.